Arthritis Drugs: Side Effects Of Shelved Medication Include Bone Decay, Joint Deterioration
Two years ago a series experiments to develop a new prescription drug that would successfully alleviate the pain caused by arthritis, conducted by some of the world's biggest pharmaceutical companies, was put on hold by the United States' Food and Drug Administration (FDA) after reports revealed that the drugs were causing bone decay and joint deterioration. On Monday, March 12, Pfizer Inc., Johnson & Johnson and Regeneron Pharmaceuticals will argue that they should continue to study the drug, while taking the necessary safety precautions to protect their patients.
The FDA's proposed questions seem to attempt to continue to keep the companies from moving forward with their experiments and limit future testing.
Considering what is known thus far about the risks and benefit associated with this class of biologic agents, are there any populations for which further clinical development would be acceptable? asks one agency discussion question, according to AP.
If the drugs are approved by the FDA their use may be more narrowly defined than originally intentioned.
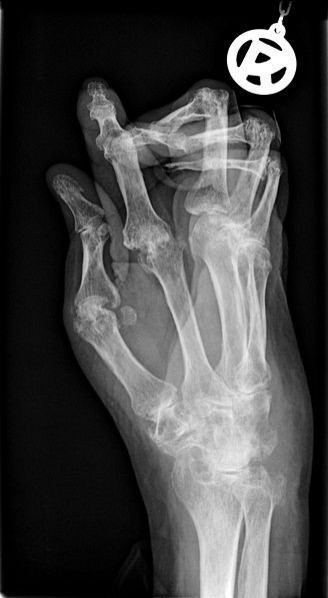
The drugs were once touted by pharmaceutical companies. They are known as nerve-growth-factor inhibitors, and considered to be potential breakthrough for treating osteoarthritis, back pain and chronic pain conditions. For over a century these symptoms have been treated by painkillers like aspirin, Advil or powerful opiates, but these can be problematic. Advil can cause stomach bleeding while opiates are extremely addictive.
The injectable nerve-silencing drug would offer a new approach to blocking the proteins that control sensation in the body. But problems with the new drug emerged in the summer of 2012. First Pfizer halted studies in June after the FDA received reports that osteoarthritis got worse in some patients, causing joint failure. Then, in Dec., 2010, the FDA put a hold on all research on the class of drugs.
The FDA says there is a clear connection between the nerve-blocking medications and incidences of joint failure which caused the FDA to shut down studies of the drug in 2010. These side effects were less common when the drug was used in low doses, which means it may be possible to reintroduce it to the market under specific guidelines.
FDA released its safety analysis to the public on Thursday, March 8, ahead of Monday's public meeting.
This request to start studies up again is unusual. Pharmaceutical companies often abandon experimental research when safety becomes an issue. But considering how widespread arthritis is (more than 50 million American adults have been diagnosed with it, or 20 percent) the potential health gain--and possible financial gain, as well--is too great to ignore.
Drug makers will likely argue on Monday that the reported joint deterioration is caused by rare drug side effects. According to reports from both Pfizer and J&J, bone deterioration only occurred in patients taking the experimental drugs in addition to traditional anti-inflammatory painkiller.
The FDA's own reports support this theory. However, they also note that Pfizers version of the drug, tanezumab, caused significant bone problems even when used on its own.
© Copyright IBTimes 2024. All rights reserved.





















