House Passes Bill To Hasten New Medical Treatments To Market
A bipartisan bill overwhelmingly passed by the U.S. House of Representatives Friday could permit some pharmaceutical companies to earn approval of new medicines and medical devices on the basis of smaller and shorter trials or by pointing to observational evidence not previously considered scientifically robust. Advocates say their legislation, passed by a 344-77 vote, would bring new lifesaving treatments to patients more cheaply and quickly. But opponents charge the measure would open the door to medical products of inferior quality.
The 21st Century Cures Act allows companies working on drugs for rare conditions to use smaller trials than were previously required, while expanding the use of patient registries to find trial subjects with less effort and expense. Device manufacturers could use shorter and smaller trials in their evaluations, too. Companies seeking to find more uses for existing medicines could submit case studies to support their claims that the drugs work for new diseases. These and other measures are intended to make the drug-development process more efficient and cheaper for companies, which expressed broad support for the bill.
“21st Century Cures is one of the most important bills we are considering this Congress because it can actually save lives,” House Majority Leader Kevin McCarthy, R-Calif., said in a statement shortly after the vote Friday. “This bill preserves America’s place at the forefront of medical innovation by making a commitment to fund medical research, break down barriers to collaborations, and cut the time it take to get innovations from the laboratory to the doctor’s office.”
It’s no secret that until last year, the pipelines of pharmaceutical companies were in a prolonged slump -- the number of new treatments that earned approval from the Food and Drug Administration per billion U.S. dollars invested in drug development was halved about every nine years from 1950 to 2012. In 2014, that worrisome streak was broken and approvals hit an 18-year high with 41 new drugs.
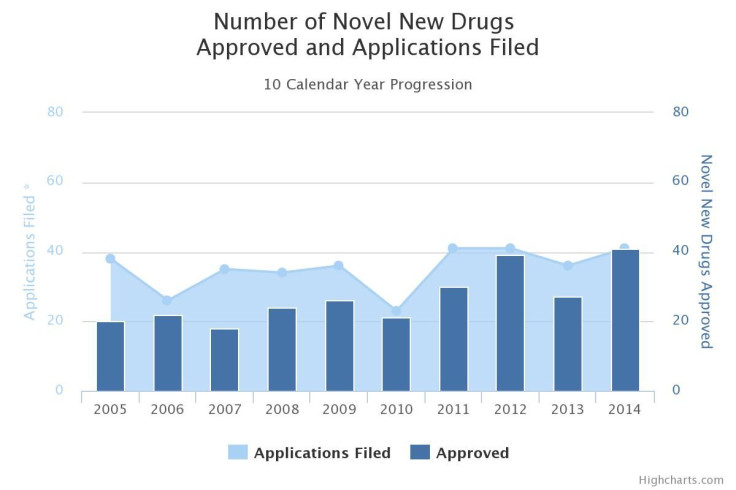
Still, many health care professionals and public health officials say it’s not enough, particularly given the persistent lack of new medicines in critical areas such as antibiotics and the industry shift toward lucrative treatments that work only in a small number of patients.
But Dr. Jerry Avorn, a professor of medicine at Harvard University, argues in a piece published recently in the New England Journal of Medicine that drug development is already quite efficient. He notes the FDA evaluates most new drug applications within six to 10 months of their submittal. Furthermore, one-third of newly approved drugs today are based on evidence from one trial with a median size of 760 patients, which Avorn considers small. The studies that underlie two-thirds of drug approvals also ran for six months or less, which he points out may be too short to catch long-term side effects in patients who are required to take a drug for years.
“Patients and physicians would not benefit from legislation that, instead of catapulting us into the future, could actually bring back some of the problems we thought we had left behind in the 20th century,” he writes.
The House Energy and Commerce Committee unanimously approved the bill in May, and opponents within the House had largely focused on how to fund it.
The funding question is a substantial one -- the bill would provide $8.75 billion over the next five years to the National Institutes of Health, which invests in scientists who do basic research that often leads to new treatments. The FDA, overseer of the safety and efficacy of new products, would receive an additional $550 million.
© Copyright IBTimes 2024. All rights reserved.












