3D-Printed Organs Could Soon Be Real With Bio-Ink Breakthrough
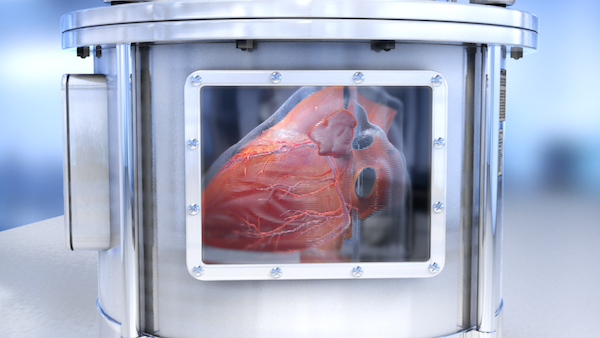
A group of researchers have made a breakthrough in 3D printing by creating a bio-ink that can piece complicated biological structures with different kinds of cells, paving way for 3D printed organs that could revolutionize healthcare.
3D printing has revolutionized manufacturing. What was predicted to be a slow merger has gathered momentum and 3D printing devices are now sold in retail where anyone can go pick one up and start making customized products. From houses to ears, scientists have successfully used 3D printing to create a plethora of things. Scientists even created a 3D printed working grenade launcher called RAMBO recently. As this technology advanced, scientists started dabbling in 3D printed life.
Using just printers, doctors replaced a person’s skull, made a human ear and even reconstructed faces.
Scientists are now ready to take the next step and create 3D printed organs that can replace organs in the body and perform the same function. Though this idea seems outlandish and very science fiction-like, we are moving closer to this technology becoming a reality every day.
This process called bioprinting is taking the medical world by storm. Scientists have already recreated blood capillary networks and even the human heart from a printing machine. But, bioprinting still faces many technical challenges.
There have been many advances but the bio ink itself is still convoluted science. Processing the bio-ink and making it stick to itself and hold the desired printed gel structure are proving particularly difficult, especially in inkjet printing.
Several methods exist that can create bio-ink droplets that can be glued together to make tissues and capillaries but the issue is that this was not perfected for every type of cell, which is integral when making organs, like the liver, which have many working parts and different kinds of cells.
Researchers from Osaka University have now refined an enzyme-driven approach to sticking biological ink droplets together, which can put together complex tissue systems required for printing biological systems.
"Printing any kind of tissue structure is a complex process. The bio-ink must have low enough viscosity to flow through the inkjet printer, but also needs to rapidly form a highly viscose gel-like structure when printed. Our new approach meets these requirements while avoiding sodium alginate. In fact, the polymer we used offers excellent potential for tailoring the scaffold material for specific purposes," Lead author, Shinji Sakai, said in Phys.org release.
All existing methods use sodium alginate as the binding agent to give this ink the unique viscosity it needs. This is popularly used in inkjet bioprinting, but has some compatibility problems with certain cell types.
The researchers developed a new method using hydrogelation mediated by an enzyme, horseradish peroxidase (found in the root of horse radish), which can create cross-links between phenyl groups of an added polymer in the presence of the oxidant hydrogen peroxide.
The team had to tip-toe around hydrogen peroxide as it is damaging to cells. This common disinfectant was introduced in drops and the cells were introduced separately to minimize contact. According to the release, “more than 90 percent of the cells were viable in biological test gels prepared in this way. A number of complex test structures could also be grown from different types of cells.”
"Advances in induced pluripotent stem cell technologies have made it possible for us to induce stem cells to differentiate in many different ways," co-author Makoto Nakamura said. "Now we need new scaffolds so we can print and support these cells to move closer to achieving full 3-D printing of functional tissues. Our new approach is highly versatile and should help all groups working to this goal."
They study was published in journal Macromolecular Rapid Communications .
© Copyright IBTimes 2024. All rights reserved.











