Birth Control Pills Recalled by Glenmark Generics
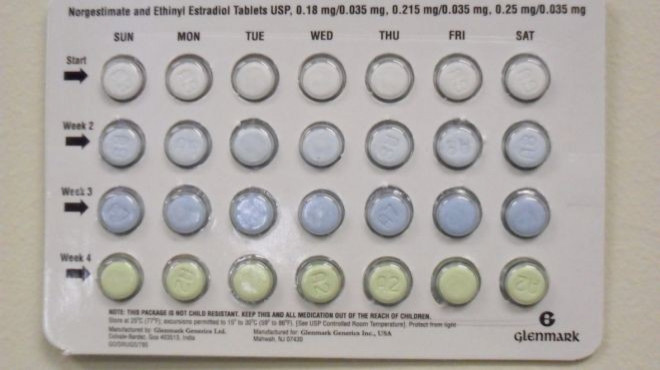
Drug maker Glenmark Generics recalled birth control pills due to a packaging error that could make the pills ineffective. The pills are labeled as norgestimate and ethinyl estradiol tablets. The India-based company issued the recall on Friday.
The packaging contains four different colored pills. The recall came about because the colors in three lots are out of order. Three colors have varying amounts of active ingredients and a fourth contains an inactive ingredient. In correct packets, there are seven white to off-white tablets in the top row and seven green tablets on the bottom row.
As a result of this packaging error, the daily regimen for these oral contraceptives may be incorrect and could leave women without adequate contraception, and at risk for unintended pregnancy, a Glenmark spokesman said in a statement.
Affected lot numbers are:
- 04110101
- 04110106
- 04110107
- 04110114
- 04110124
- 04110129
- 04110134
Glenmark distributed the pills between September and December with expiration dates between July and September 2013. Packets with an affected lot number or any packet with the lot number or expiration date not visible is subject to the recall and should be returned to a pharmacy.
Any patient with the affected product should switch to a non-hormonal method of contraception such as condoms, a Glenmark spokesman said in a statement.
This is extremely disturbing, Steven Goldstein, an obstetrician-gynecologist at New York University Langone Medical Center and Professor at the NYU School of Medicine, told Agence France-Presse.
This problem with generics manufactured outside of the USA is of great concern to me as a clinician. I often allow and encourage patients to try generics as long as they do not have nuisance side effects. However I have always assumed them of equal quality control to the branded products.
This recall is the second major birth control pill recall this month. Pfizer recalled approximately 1 million packets of Lo/Ovral-28 and its generic form on Feb. 1 when they found some packets contained wrong numbers of inert or active tablets and had some tablets out of sequence.
Another recall was issued by pharmaceutical company Qualitest in September over the same problem.
© Copyright IBTimes 2024. All rights reserved.





















