COVID Test Recall 2021: Quidel Recalls Lyra SARS-CoV-2 Assay Tests Over Inaccurate Test Results
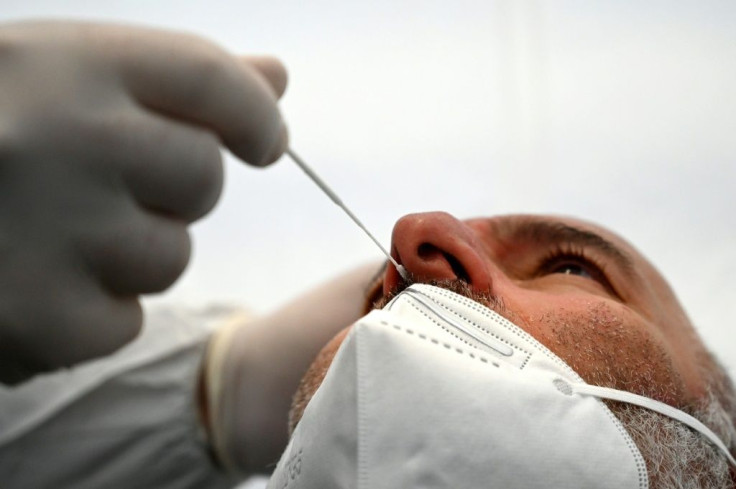
Quidel has recalled several lots of its COVID tests because they produce false-negative test results for people that have relatively high amounts of the coronavirus infection during sampling.
The Food and Drug Administration has identified the recall as a Class 1 recall, which it said is the “most serious type of recall” as the “use of the devices may cause serious injuries or death.”
The affected COVID tests were manufactured from March 17, 2020 to March 12, 2021, and distributed from March 17, 2020 through May 27, 2021. Each kit contains 96 reactions. All affected lot codes can be viewed here.
The Lyra SARS-CoV-2 Assay test is a real-time polymerase chain reaction (RT-PCR) test that uses nasal swabs to detect COVID-19.
The recall was issued after five complaints about false-negative test results from the COVID test. However, there were no reports of injuries or death from the associated recalled device.
Quidel said in its recall that the false-negative results could lead to a delayed diagnosis or inappropriate treatment of COVID that could result in harm, serious illness, or death. It could also cause the virus to spread to others if a person believes that they are negative for COVID-19.
Quidel said it has sent an “urgent” letter to all affected customers that had the test in their possession in April, requesting samples to be retested as needed.’
Questions about the recalled COVID test can be directed to Quidel Customer Service at 1-800-974-1517 (in the U.S.) or 1-858-552-1100 (outside the U.S.), Monday through Friday, from 8 a.m. to 5 p.m. ET or by email at customerservice@quidel.com.
© Copyright IBTimes 2024. All rights reserved.




















