Covid Vaccine For Kids Under 5: Everything To Know About Pfizer's FDA Plan
The Food and Drug Administration expects Pfizer to inquire about authorizing the COVID-19 vaccine for children under the age of 5.
COVID cases within children have increased dramatically since the spread of the highly contagious Omicron variant, according to the BBC. The variant makes up essentially all the new reported infections in the U.S., according to the Centers for Disease Control and Prevention.
Pfizer said in December that the low-dose test it conducted for children produced mixed results, thus implying that perhaps three doses are needed. The FDA has reportedly called on the pharmaceutical giant to present its findings for its two-dose trial so it can begin evaluating it for emergency use.
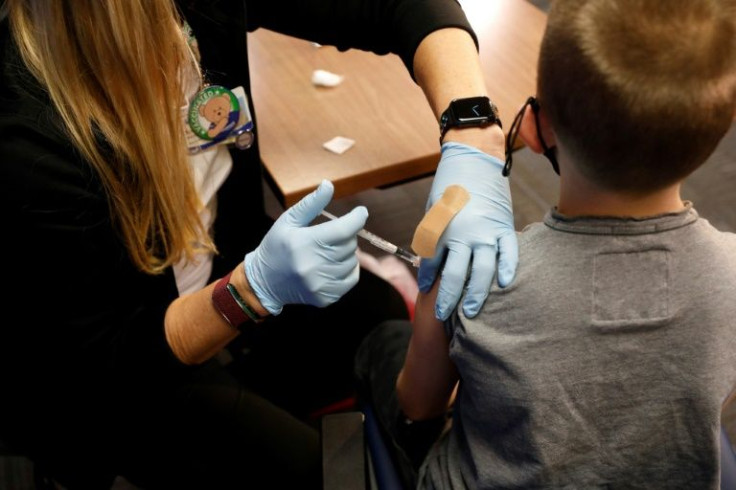
On Oct. 29, 2021, the FDA approved the emergency use of the Pfizer vaccine to include children 5 to 11 years of age, finding that the immune response of kids was comparable to people aged 16 to 25.
Pfizer expects $29 billion from vaccine sales in 2022, according to Reuters. Johnson & Johnson estimates that its one-dose COVID-19 vaccine will bring in up to $3.5 billion in sales this year.
In a statement released on Dec. 16, 2021, the CDC highlighted its “clinical preference for individuals to receive an mRNA COVID-19 vaccine [Pfizer or Moderna] over Johnson & Johnson’s COVID-19 vaccine.”
© Copyright IBTimes 2024. All rights reserved.





















