Doctors Without Borders Launches Campaign Demanding Johnson & Johnson, Mylan, And Otsuka Lower The Price Of Tuberculosis Medicine
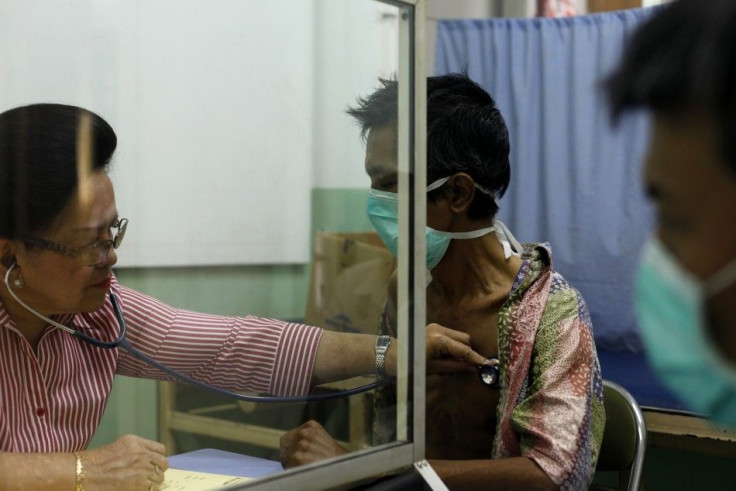
Doctors Without Borders has taken aim at Johnson & Johnson and two other drug makers demanding they lower the price of pharmaceuticals developed with tax dollars to treat drug-resistant tuberculosis.
, demanding it lower the price of a tuberculosis medicine to $1 a day so more patients can be treated.
The organization, known internationally as Médecins Sans Frontières (MSF), announced it would launch a campaign against J&J to get the company to lower the cost of bedaquiline to $1 day from the current $400 for a six-month regimen.
Bedaquiline treats multidrug-resistant tuberculosis (MDR-TB) and is typically sold under the name sirturo.
“Bedaquiline was developed using taxpayer money and contributions from the global TB community,” HIV & TB policy adviser for MSF’s Access Campaign Sharonann Lynch said in the press release. “Those who contributed to bedaquiline’s development should have a say in how the drug is priced.
"We’re calling on J&J to price bedaquiline at no more than $1 per day so that it can be made available to all people with drug-resistant TB. We will not back down until the price of bedaquiline is brought down.”
MSF said bedaquiline has served as a replacement to previous treatments for multidrug resistant TB, which could have required almost 20 pills a day and several shots. The older treatments only cured 55% of people suffering from MDR-TB and 34% of people with extensively drug-resistant TB (XDR-TB).
“We have seen so many patients go deaf, lose their jobs, or lose their lives because they had no option other than the excruciating TB drugs that had to be injected,” said Pilar Ustero, medical adviser for MSF’s Access Campaign. “Bedaquiline is proving to be a game changer, giving those people with [drug resistant]-TB who can access it a better chance to be cured, without the toxic side effects. We need this drug to be affordable for everyone who needs it, everywhere.”
MSF said research from the University of Liverpool indicate production costs for the drug could be lowered to 25 cents per day if 108,000 treatments were sold every year.
A second press release took aim at Mylan and Otsuka over the cost of TB drug delamanid, for which Otsuka owns the patent.
Delamanid is among the most expensive TB medicines on the market, costing an average of $1,700 for a six-month treatment from the Global Drug Facility. According to the MSF, the cost has greatly limited availability of the drug. As of August, around 2,900 people suffering from drug-resistant TB have received delamanid treatments, according to the DR-TB Scale-Up Treatment Action Team.
Mylan previously announced a lower price comparatively, with a six-month treatment costing $940 starting in June 2020. However, MSF said the reduction is inadequate.
“Even at $940, delamanid remains one of the most expensive DR-TB drugs, and its high price will continue to have a chilling effect on the scale-up of this drug in national TB programs,” MSF Medical Adviser Dr. Stobdan Kalon said in the release. “As long as these new drugs are priced out of reach for TB programs, an all-oral treatment regimen for people everywhere with pre-XDR and XDR-TB and children with MDR-TB will remain a distant reality and people will continue to suffer the devastating side effects of older drugs that have to be injected, including permanent deafness. Otsuka and Mylan must further drop the price of delamanid to ensure that TB programs are able to afford and scale-up the use of all-oral treatment regimens.”
© Copyright IBTimes 2025. All rights reserved.





















