Early Stage Trial Of Emergent BioSolutions' Anthrax Vaccine Starts
Rockville-based biopharmaceutical company Emergent BioSolutions Inc. (NYSE: EBS) announced the start of a early-stage clinical trial for its third generation Anthrax vaccine candidate called NuThrax.
Emergent BioSolutions said the Phase 1 clinical trial was in support of the U.S. government's multiple product strategy to strengthen the nation's biodefense capabilities.
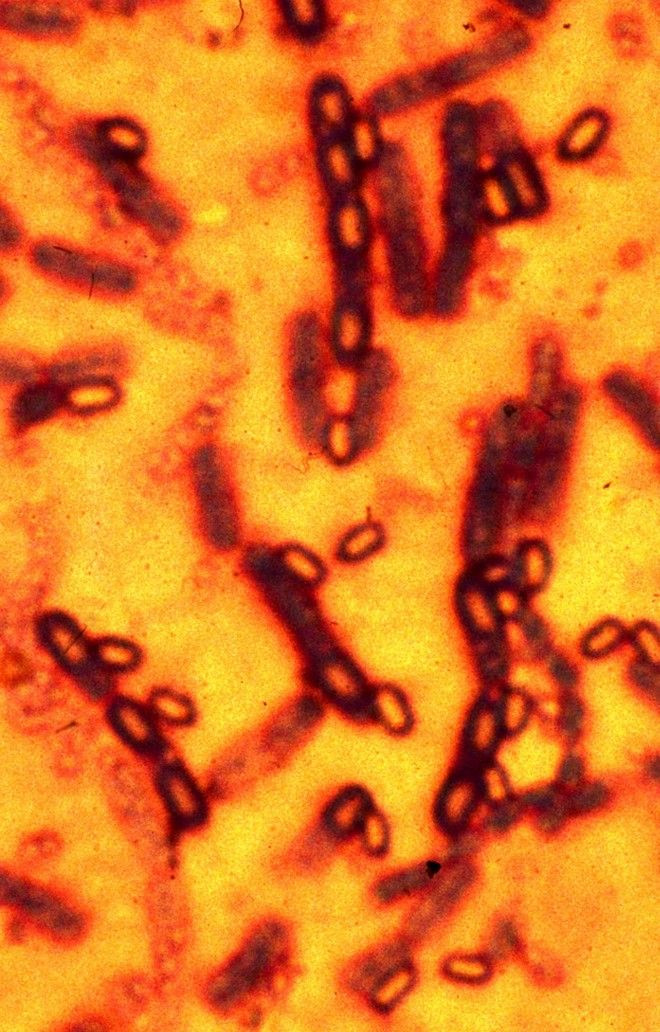
Anthrax is an acute infectious disease caused by the spore-forming bacterium Bacillus anthracis, a microbe that lives in soil. Anthrax spores can be used as a bioterrorist weapon, as was the case in 2001, when when someone purposely spread anthrax through the postal system, causing 22 cases of anthrax, including 5 deaths.
NuThrax, a combination of the company's anthrax vaccine BioThrax and a new compound, is being developed as part of Emergent's anthrax franchise. BioThrax is the only FDA-licensed vaccine available for pre-exposure protection against anthrax infection. To date, more than 10 million doses of BioThrax, commonly called Anthrax Vaccine Adsorbed (AVA), have been administered to more than 2.5 million individuals.
BioThrax is indicated for the active immunization of individuals between 18 and 65 years of age at high risk of exposure to anthrax. BioThrax is not licensed for use in a post-exposure setting.
NuThrax has the potential to exhibit advanced characteristics such as requiring fewer doses, generating an enhanced immune response and having a favorable shelf life, the company said.
If successful, this could be an attractive candidate for the government's growing arsenal of medical countermeasures, said chief operating officer Daniel Abdun-Nabi.
The company expects preliminary data from this study to be available in the third quarter of 2011.
Shares of the company, which rose to a 22-month high on Tuesday, are trading 4 cents higher at $23.71 at 09:42 am EST Wednesday on the NYSE. In the past 52 weeks, the shares have been trading in the range of $13.22 to $23.76.
© Copyright IBTimes 2024. All rights reserved.





















