Ebola Vaccine: GlaxoSmithKline Drug Could Be Ready By The End Of 2014
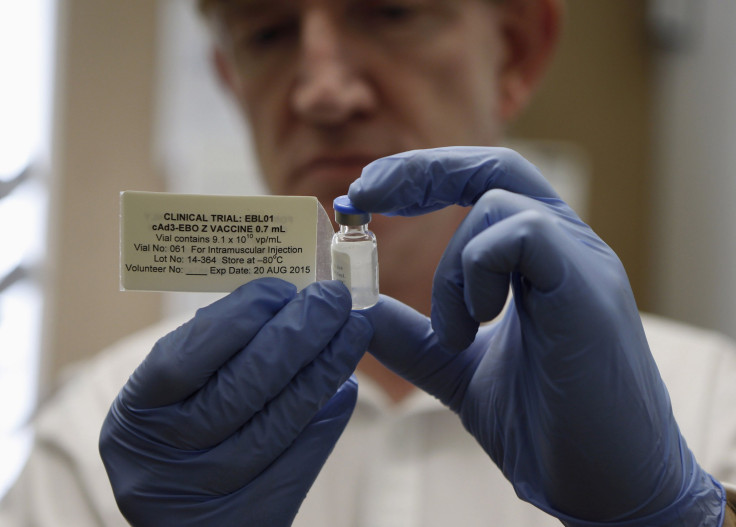
GlaxoSmithKline's Ebola vaccine could be ready by the end of the year and in use in West Africa by January 2015, Reuters reported. The London-based pharmaceutical giant acquired the drug when it purchased the biotech company Okairos in 2013.
"With the current pressing public health emergency caused by spread of the Ebola virus in West Africa, I want to report to shareholders that we are working hard to develop a potential vaccine. This is still at an early stage, but we are grateful for the support of all our partners, including the WHO, to expedite development of this candidate vaccine," GSK CEO Andrew Witty said in the company's third-quarter announcement. GSK and the National Institutes of Health developed the experimental Ebola vaccine and the drug entered the Phase 1 clinical trial stage, testing its ability to develop an immune system response in humans and safety, in August.
In addition to the GSK/NIH Ebola vaccine, NewLink Genetics Corporation is developing the VSV-EBOV drug. And the company shipped the first of 800 vials of the vaccine to the World Health Organization Monday.
The GSK Ebola vaccine is different from VSV-EBOV, as it is based on a chimpanzee cold virus, chimp adenovirus type 3 (ChAd3), which is used as a "carrier" to deliver genetic material from the Zaire Ebola and Sudan Ebola strains. "The vaccine candidate delivers one part of Ebola’s genetic material to human cells, but the adenovirus vector [chimpanzee cold virus] does not replicate. Rather, the Ebola gene that it carries allows the cells of the vaccine recipient to express a single Ebola protein, and that protein prompts an immune response in the individual. It is important to know that the Ebola genetic material contained in the investigational vaccine cannot cause a vaccinated individual to become infected with Ebola," the NIH explains.
Witty said the first batch of GSK's Ebola vaccine could soon be shipped to the WHO and could be the first vaccine to be used in West Africa, Reuters reported. "I fully anticipate that the initial supply should be available before the year-end," Witty said to reporters during a third-quarter results press conference. An earlier report indicated a possible collaboration between GSK and Johnson & Johnson, which is developing its own Ebola vaccine. The two companies could work to develop a vaccine and boost production.
In addition to GSK's Ebola vaccine and VSV-EBOV, serums made from the blood of people who have survived Ebola could be available within weeks in West Africa, reports BBC. If a person has successfully fended off the infection, it means that they have antibodies in their blood that can attack Ebola.
© Copyright IBTimes 2024. All rights reserved.






















