FDA Approves Synthetic Surfactant to Treat Life-Threatening Condition in Pre-Term Babies
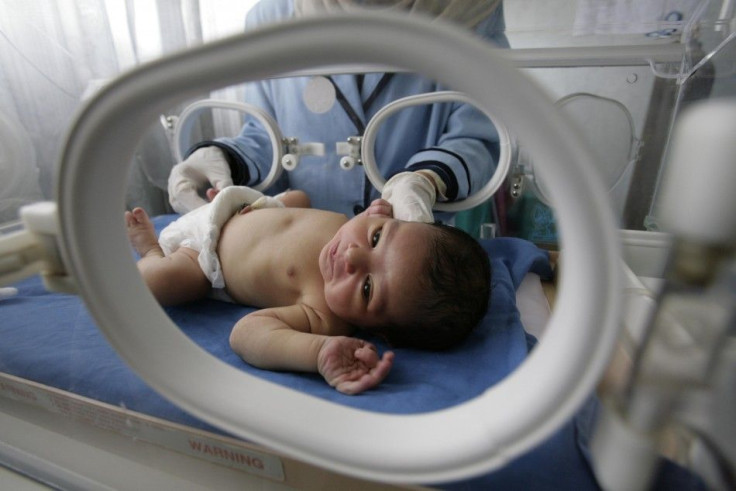
The Food and Drug Administration (FDA) has approved Surfaxin (lucinactant) to treat Respiratory Distress Syndrome (RDS), a life-threatening condition affecting pre-term babies.
Surfaxin (lucinactant intratracheal suspension) is the first FDA- approved synthetic peptide- containing surfactant, claims Discovery Labs, Warrington, PA, which played a major role in developing the surfactant.
RDS affects premature babies as they are born prior to the time surfactant is produced in their lungs. The more premature a baby is the more likely it will suffer from the respiratory disease.
Surfactant is a liquid that coats the inside of the lungs. It helps keep the air sacs open and breathe normally. A low surfactant level makes the lungs collapse and brings the oxygen levels in the body down.
Mechanical ventilation and animal-derived surfactant replacement therapy are some of the treatments adopted for the disease currently.
Animal surfactants are normally derived from cow or pig lungs and are very expensive. They contain some material harmful to the lungs and cannot be produced in large scale.
The new synthetic surfactant is not immunogenic and is better as the animal-derived surfactant can be used only once as it causes immune reaction.
Imitating a natural peptide, known as Surfactant Protein B, inventor Susan Revak and Cochrane Lab developed a synthetic version of surfactant in the 1990s.
The Scripps Research Institute conducted some formative work in the new version.
I am excited that our scientific findings will help save lives, said Charles Cochrane, M.D., professor emeritus at Scripps Research. Many years of work in our basic research laboratory at the Scripps Research Institute made this landmark development possible.
Discovery Labs supervised the three phases of clinical trials required by the FDA, a Scripps Research press release said.
The approval of SURFAXIN is an important medical advancement for the neonatology community and parents of preterm infants who will soon have an effective alternative to animal-derived surfactants to prevent the development of RDS, said W. Thomas Amick, Chairman of the Board and Chief Executive Officer of Discovery Labs.
This is a significant milestone in our continuing efforts to develop a pipeline of products to further advance the standard of respiratory critical care, he said.
Discovery Labs hopes to launch Surfaxin in the U.S. later this year.
© Copyright IBTimes 2024. All rights reserved.





















