FDA Authorizes First At-Home COVID-19 Collection Kits; Available Next Few Weeks
KEY POINTS
- FDA authorized coronavirus at-home sample collection kits
- FDA partnered with LabCorps
- Contains Q-tip cotton swab and saline
- Collect samples at home; mail to LabCorp
- Available in next few weeks with doctor's orders
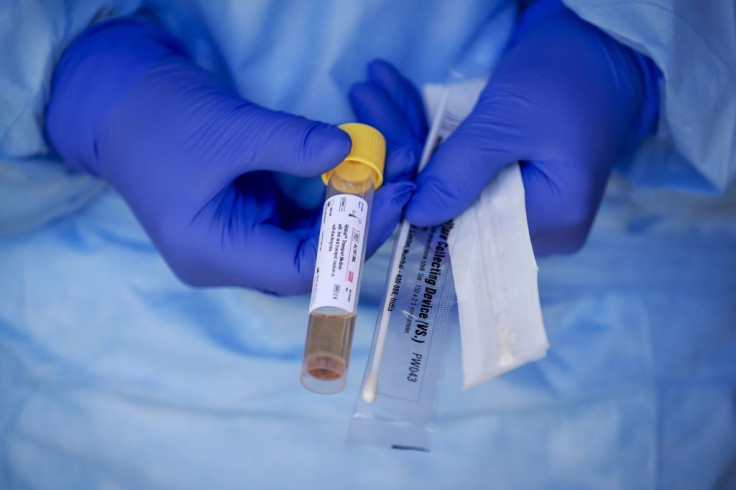
The U.S. Food and Drug Administration announced that emergency use authorization of the first at-home coronavirus kits which would be made available in the next few weeks.
According to the New York Post, the FDA granted the emergency use authorization of the coronavirus at-home diagnostic tests which would allow individuals to collect their own samples and mail them to a laboratory.
FDA granted this authorization to LabCorp, which planned to make their Pixel by LabCorp COVID-19 Test home collection kits available within the next few days as long as they are accompanied with a doctor’s order.
#BREAKING LabCorp has received emergency use authorization from the FDA for a #COVID19 at-home test kit https://t.co/bwAbwg5mDm
— ABC7 Eyewitness News (@ABC7) April 21, 2020
“Throughout this pandemic we have been facilitating test development to ensure patients access to accurate diagnostics, which includes supporting the development of reliable and accurate at-home sample collection options,” FDA Commissioner Stephen Hahn, M.D. said in a statement. “The FDA’s around-the-clock work since this outbreak began has resulted in the authorization of more than 50 diagnostic tests and engagement with over 350 test developers.”
The kit contains a Q-tip style cotton swabs and saline which would allow users to collect samples from their noses and mail them to LabCorp for testing.
Due to concerns regarding cross-reactivity and sterility because of the inherent genetic materials in cotton swabs, it is inadvisable to use other cotton swabs for these tests as of the moment.
The FDA is currently working with the test developers to see if the Q-tip style cotton swab is safe and effective to use in other tests.
Hahn said that they worked with LabCorp specifically for the tests that include home sample collection to make sure that the data shown from the at-home patient sample collection is as safe and accurate as the samples collected by a doctor, the hospital or by other testing sites.
The agency emphasized that the authorization is not a general sanction to collect patient samples at home using other “collection swabs, media or tests”, or for full at-home testing.
LabCorp, which was based in North Carolina, launched its launched its laboratory tests for the COVID-19 in March and currently conducts around 65,000 tests a day with results being released within two to four days, Reuters reported.
© Copyright IBTimes 2024. All rights reserved.





















