How To Get Your Marijuana Trial FDA Approved

The same day that the New York State legislature passed a bill that will make it the 23rd state in the nation to legalize medical marijuana, the Food and Drug Administration also took steps on Friday to bolster support for clinically researching medical uses for the drug.
The FDA, the agency which oversees the use of food and drugs, has for the first time outlined the process for getting federal approval to conduct research with marijuana, making it the first of its kind to be readily available online. A spokesman for the FDA said this measure has been taken to ensure the administration is transparent about the process as well as supporting researchers who wish to pursue marijuana research.
“There is no fundamental change in the way our approval process works,” Jeff Ventura, a spokesman told IBTimes, explaining that it has simply been publicized so people can understand the policy. In addition, the agency reiterated its backing for studies that fall in line with the drug approval process.
Ventura stated that the administration “supports those in the medical research community who intend to study marijuana in scientifically valid investigations.”
Attempting to get any clinical trial request reviewed is fairly complex and full of bureaucratic department-hopping but for a Schedule I drug like cannabis -- which is strictly regulated along with drugs like heroin or methamphetamine -- the process is even more convoluted.
If someone wants approval to conduct research with marijuana, they will have to work with three different federal departments in a process that can take months: Potential researchers start with the FDA, then contact the National Institute on Drug Abuse (NIDA) and finally the Drug Enforcement Administration (DEA) only to continue going back and forth between the departments until the researchers get a stamp of approval from the FDA and their government-approved pot.
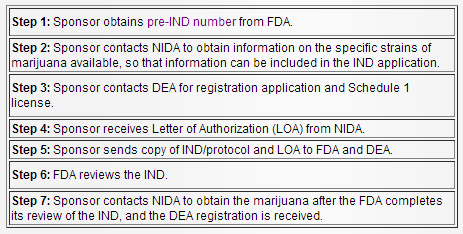
The long, bureaucracy-intensive process ensures that the FDA approves of marijuana trials that occur in “well-controlled clinical trials,” according to Ventura.
Ventura said the FDA is regularly meeting with researchers on developing new drugs that are derived from marijuana, though he could not say if the FDA expects an increase in clinical research requests by publicizing the process.
© Copyright IBTimes 2024. All rights reserved.






















