Pfizer Birth Control Recall for Pregnancy Risk: Are You Affected? How to Tell
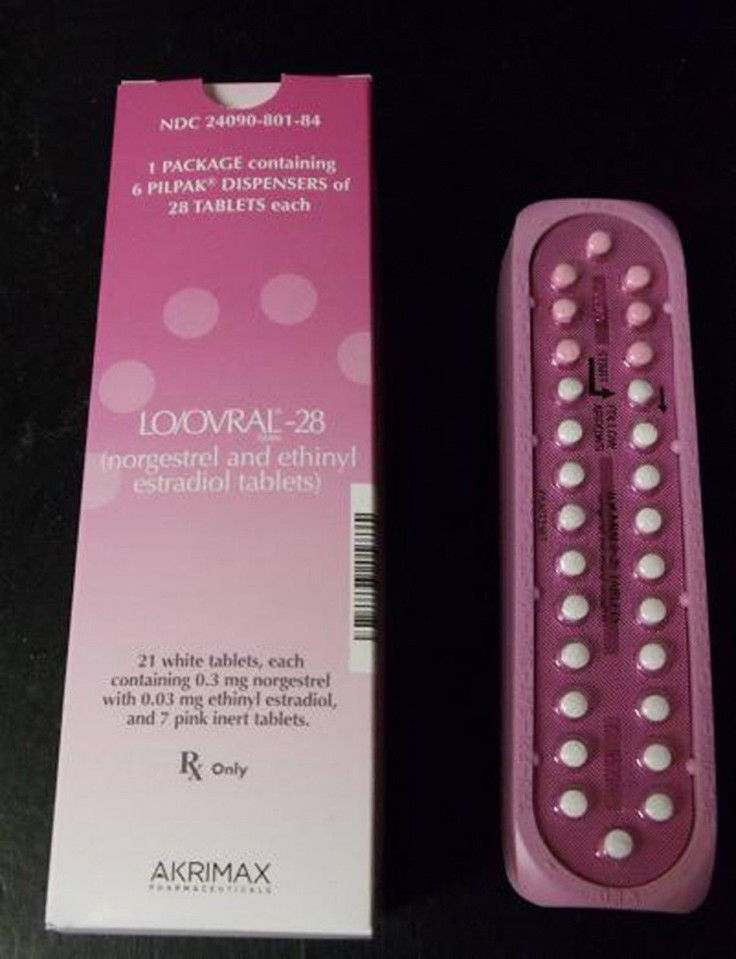
As word spread about the mass birth control recall of at least one million pill packets in the U.S. from Pfizer on Tuesday, panic ensued amongst women nationwide about their risk of becoming pregnant.
According to Pfizer, an error in packaging from the inspection department lead to the recall, as the affected birth control pill packets contain an incorrect amount of active tablets or are out of order, which do not allow the medication from preventing pregnancy effectively.
As a result of this packaging error, the daily regimen for these oral contraceptives may be incorrect and could leave women without adequate contraception, and at risk for unintended pregnancy, said Pfizer, in a statement on the U.S. Food and Drug Administration Web site.
The world's largest drug maker said that there are no health or safety risks involved with the recall, but women using the prescription should immediately use another, non-hormonal method of contraception.
One the recall was issued, widespread panic occurred between women on social media Web sites like Facebook and Twitter scrambling to find out if their prescription was part of the recall. Taking a step further, law offices nationwide began to roll out advertisements to chase potential clients affected by the recall with health problems or unplanned pregnancy.
However, the birth control pills under the recall are not the most commonly prescribed brands of birth control used by women, despite the fact that Pfizer is the world's largest drug maker. According to The Associated Press, the brand affected, Lo/Ovral, ranked 64th in sales in the U.S. last year while the generic version, norgestrel and ethinyl estradiol, ranked 30th based on data from IMS Health.
The AP said Lo/Ovral isn't as widely prescribed, according to doctors, so they do not expect a flood of panicked phone calls, though some confidence in the method will diminish, according to a spokesperson at Planned Parenthood.
Unfortunately, this manufacturing error diminishes people's confidence in an extremely important and safe method of contraception, vice president for external medical affairs at Planned Parenthood Dr. Vanessa Cullins told the Baltimore Sun.
Company spokeswoman Kristen Neese said Pfizer learned of the problem in December when a customer pointed it out, The AP reported. The recall was issued to pharmacies immediately after but did not announced the recall publically until Tuesday after being prompted by the Food and Drug Administration.
So how do you know if the birth control pills you are taking are part of the recall?
1. Brand
Not all brands of birth control have been recalled. The affected pills are 14 lots of Lo/Ovral-28 tablets and 14 lots of generic Norgestrel and Ethinyl Estradiol tablets.
2. Expiration Dates
The recall is for expiration dates ranging from July 31, 2013, to March 31, 2014.
(For more details regarding the birth control recall, click here for a full list or scroll below.)
3. Location
The pills were distributed to warehouses, clinics and retail pharmacies nationwide, so any pharmacy in the U.S. could have had stock of the recalled product.
If you are unsure, contact your doctor or pharmacy immediately. Pfizer also issued a statement that consumers should report any adverse effects to Akrimax Medical Information at 1-877-509-3935 (8 AM to 7 PM Mon-Fri CST) or to FDA's Med Watch Program.
Here is a full list of the birth control pills affected by the Pfizer recall, courtesy of the Pfizer Web site.
NDC
Product
Lot
Expiration
Configuration/Count
24090-801-84
LO/OVRAL® 28
E15678
08/31/2013
6 Pilpacks® of 28 tablets each
24090-801-84
LO/OVRAL® 28
E15679
08/31/2013
6 Pilpacks® of 28 tablets each
24090-801-84
LO/OVRAL® 28
E15686
08/31/2013
6 Pilpacks® of 28 tablets each
24090-801-84
LO/OVRAL® 28
E15687
01/31/2014
6 Pilpacks® of 28 tablets each
24090-801-84
LO/OVRAL® 28
E15690
01/31/2014
6 Pilpacks® of 28 tablets each
24090-801-84
LO/OVRAL® 28
E15698
01/31/2014
6 Pilpacks® of 28 tablets each
24090-801-84
LO/OVRAL® 28
E15700
02/28/2014
6 Pilpacks® of 28 tablets each
24090-801-84
LO/OVRAL® 28
E80434
07/31/2013
6 Pilpacks® of 28 tablets each
24090-801-84
LO/OVRAL® 28
E80438
08/31/2013
6 Pilpacks® of 28 tablets each
24090-801-84
LO/OVRAL® 28
F36908
02/28/2014
6 Pilpacks® of 28 tablets each
24090-801-84
LO/OVRAL® 28
F36909
02/28/2014
6 Pilpacks® of 28 tablets each
24090-801-84
LO/OVRAL® 28
F43915
03/31/2014
6 Pilpacks® of 28 tablets each
24090-801-84
LO/OVRAL® 28
F43926
03/31/2014
6 Pilpacks® of 28 tablets each
24090-801-84
LO/OVRAL® 28
F43927
03/31/2014
6 Pilpacks® of 28 tablets each
24090-961-84
Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg
E15677
08/31/2013
6 Pilpacks® of 28 tablets each
24090-961-84
Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg
E15704
01/31/2014
6 Pilpacks® of 28 tablets each
24090-961-84
Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg
E15706
01/31/2014
6 Pilpacks® of 28 tablets each
24090-961-84
Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg
E80440
08/31/2013
6 Pilpacks® of 28 tablets each
24090-961-84
Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg
F16388
01/31/2014
6 Pilpacks® of 28 tablets each
24090-961-84
Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg
F16390
02/28/2014
6 Pilpacks® of 28 tablets each
24090-961-84
Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg
F22132
02/28/2014
6 Pilpacks® of 28 tablets each
24090-961-84
Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg
F31330
02/28/2014
6 Pilpacks® of 28 tablets each
24090-961-84
Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg
F36911
03/31/2014
6 Pilpacks® of 28 tablets each
24090-961-84
Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg
F36913
03/31/2014
6 Pilpacks® of 28 tablets each
24090-961-84
Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg
F43924
03/31/2014
6 Pilpacks® of 28 tablets each
24090-961-84
Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg
F43925
03/31/2014
6 Pilpacks® of 28 tablets each
24090-961-84
Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg
F43934
03/31/2014
6 Pilpacks® of 28 tablets each
24090-961-84
Norgestrel 0.3 mg/Ethinyl Estradiol 0.03 mg
F53238
03/31/2014
6 Pilpacks® of 28 tablets each
© Copyright IBTimes 2024. All rights reserved.






















