Radioactivity: What It Is
Radioactivity is when an unstable atomic nucleus changes into another, more stable form. The process is known as radioactive decay.
Radioactivity was first discovered in the 1890s, by Henri Becquerel. Marie Curie would later refine the work, winning the Nobel Prize for Chemistry in 1911. Curie was the first to name the radioactive element polonium and discovered radium as well. (Her work with radioactive elements was with no protection, and she died of cancer). Curie and Becquerel both noticed that certain elements emitted several kinds of radiation, and it was Ernest Rutherford who realized that the radioactive decay process resulted in one element transforming into another.
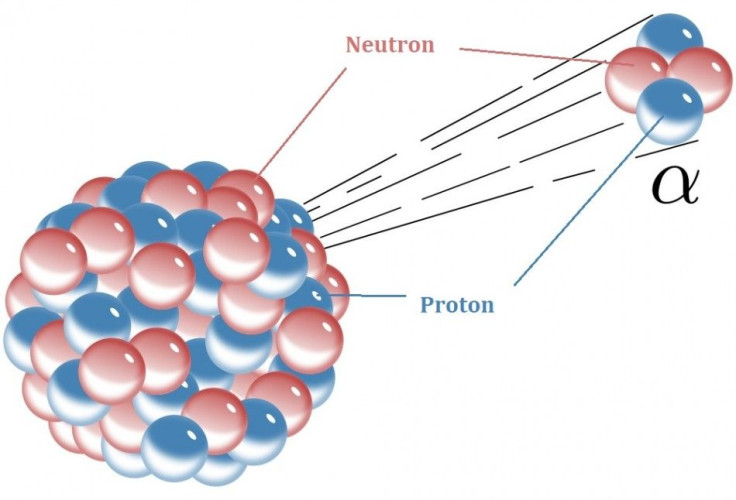
Most atoms are stable, and last essentially forever. But radioactive elements are not. All atoms are made of protons, neutrons and electrons. The number of protons determines which element it is. The number of neutrons determines which isotope it is. For an atom to be stable the nucleus has to be energetically balanced in such a way that there is no lower energy state.
One can imagine a pile of billiard balls in a pyramid: it will stay in a pyramid shape as long as there isn't a push on any of the balls. When one is disturbed, the pile falls apart into the lower energy state, which is a bunch of balls scattered on the table. Radioactive nuclei behave in a similar way: with a small push of energy in some form, the nucleus will come apart.
Sometimes this happens spontaneously. It won't always happen, but there is a certain probability over time that it will for each individual atom. For this reason atoms have half-lives, which is the time it takes for half of whatever element it is to turn into other elements, called daughter isotopes. When one says that uranium-238 has a half-life of 4.4 billion years, it means that after that time half of it will have decayed into its daughter isotopes (mostly lead).
Radioactive decay takes several forms. One is alpha decay, where the piece of the nucleus that flies off is two protons and two neutrons, which is an atom of helium (without its usual complement of electrons). The second is beta decay, in which the atom emits an electron, which is negatively charged, or its positively charged cousin, the positron. A third type is gamma emission, in which the atom gives off a highly energetic photon along with the alpha or beta particle. Another is neutron emission, which is when a neutron escapes the nucleus.
Alpha decay creates an atom that is two protons and two neutrons lighter. For example, uranium-238 (element number 92), which is used in nuclear reactors, loses an alpha particle to become thorium-234 (element number 90).
When an atom undergoes beta decay, it converts a neutron to a proton. This brings the atomic number of the element up by one, changing it. Caesium-137, (element number 55) for example, emits an electron and becomes Barium-137 (element number 56).
Neutrons once emitted aren't stable; they don't last long (they turn into protons after less than a minute). But they do sometimes hit other atoms, which in turn can change them, or send protons, electrons and photons in all directions.
In a nuclear reactor, the heat comes from the emission of neutrons from uranium-235 (U-235). The neutrons hit other uranium nuclei and they in turn split, hitting others. This energetic motion of atoms is what generates the heat that boils the water in a nuclear reactor.
The uranium doesn't last forever. While U-235 has a half-life of 703 million years, a typical piece of nuclear fuel isn't entirely made of that. It has other elements and other isotopes in it, notably U-238. U-238 isn't ordinarily very radioactive. But when hit with a neutron from a U-235 nucleus it changes into plutonium-239.
It is this process that eventually means the nuclear fuel has to be taken out and replaced. The elements in the spent nuclear fuel are largely the daughter isotopes of the original uranium, and consist of elements such as thorium and protactinium. Eventually, uranium decays into lead-207, which is stable. Among the products of nuclear fission are iodine-131, casesium-137, and strontium-90, which are of more concern to health authorities as they last long enough to be ingested or inhaled in the event of a disaster.
A certain amount of radioactivity is always present; it is when the levels go above the background for an extended period that health problems can appear. The health effects come from the particles and gamma rays hitting the atoms of your body, breaking them up, or causing them to ionize, in which case they sometimes react with other chemicals.The results are birth defects, cancers and radiation poisoning.
Shielding oneself against radiation depends on what one wants to stop. Gamma rays are highly energetic and are usually only stopped by a very large amount of matter. Lead is often used because it is dense and the photons are more likely to hit something before they reach whatever is being shielded. Alpha particles can be stopped by a piece of paper or skin. Beta particles require a thin sheet of metal. Neutrons generally have to be absorbed by something, and there are a number of elements and chemicals -- notably water -- that do the job well.
© Copyright IBTimes 2024. All rights reserved.





















