Roche Warns of Fake Avastin Cancer Drug in U.S.
Roche Holding AG, the Swiss drugmaker, warned that a counterfeit version of the widely used anti-cancer drug Avastin may have been purchased and used in a number of medical facilities in the U.S, potentially putting patients at risk.
The counterfeit product is not safe or effective and should not be used, Roche said in a joint statement with Genentech, its U.S. subsidiary.
The company said chemical analyses have showed that the counterfeit drugs do not contain the active ingredients for Avastin, which is approved by the U.S. Food and Drug Administration to treat colon, lung, kidney and brain cancers.
Roche said it is working with the FDA to find the source of the counterfeit drug and prevent it from further distribution.
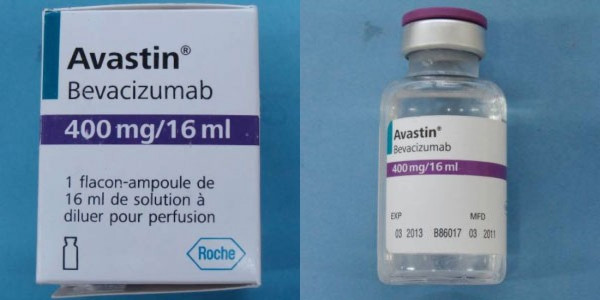
In the U.S., authentic Avastin are labeled in English, say they were made by Genentech and have a six-digit lot number with no letters. The counterfeit Avastin was packaged in boxes that had writing in French, identified Roche as the manufacture and had lot numbers on the boxes or vials starting with B86017, B6011 or B6010.
Authentic Avastin FDA-Approved for Use in the United States
Source: Roche
Counterfeit Product
Source: Roche
In a related action, the FDA said late Tuesday it sent letters to 19 medical practices known to have purchased unapproved cancer medications, including counterfeit Avastin, from a company called Quality Specialty Products, also known as Montana Health Care Solutions.
Avastin is among Roche's top-selling drugs with a turnover of 5.3 billion Swiss francs ($5.8 billion) in 2011.
Roche ADRs fell nine cents to $43.85 in midday trading.
© Copyright IBTimes 2024. All rights reserved.






















