Silicone Breast Implants Will Continue to Remain in Market: FDA
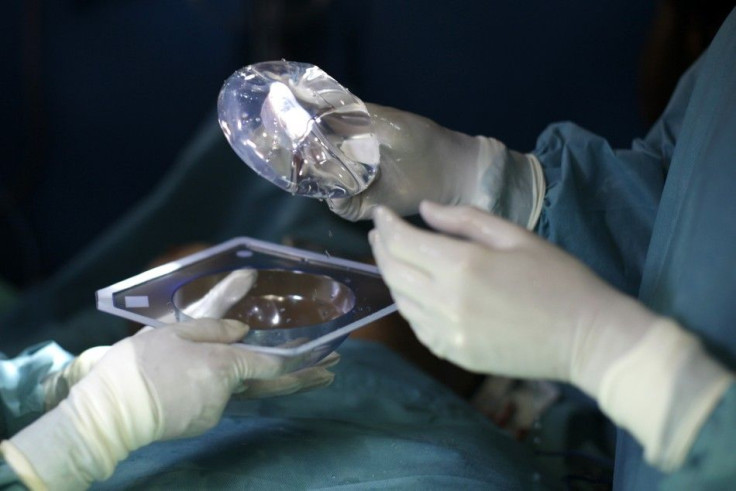
The Food and Drug Administration ruled on Thursday that silicone Breast Implants will stay in the market, after two days of discussions and hearings on the device's safety and aftereffects.
When asked about the opinion on the implant's safety, We felt that way before the meeting, and we continue to feel that way after the presentations and discussions over the past two days, Dr. William Maisel, deputy director of the FDA's Center for Devices and Radiological Health, told The New York Times.
Despite FDA's approval of the surgery for breast enhancement, critics still express their concern over the low participation in their follow-up studies on its potential to cause health issues.
Earlier in June, FDA had pointed out that women who undergo the knife experience post surgery complications frequently, and at times need additional surgery to fix or replace them. It also emphasized that Silicone implants must not be considered as lifetime devices.
Studies from two manufacturers, Allergan and Mentor, involved about 61 percent and 21 percent of patients respectively, the Wall Street Journal reported.
The FDA said it would work with the companies, both divisions of Johnson&Johnson, to get more women to take part in mandated post-approval studies, a thirdage.com report said.
Till 2006, Silicone implants were banned for more than 10 years due to concerns about leaking silicone gel and the problems it caused to the connective tissues. This year's study findings have not showed increased risk of cancer or connective tissue disease but FDA noted that may be a longer study period is needed to establish more concrete conclusions.
© Copyright IBTimes 2024. All rights reserved.





















