Two Ebola Vaccines Pass Early Safety Tests In Liberia Trial
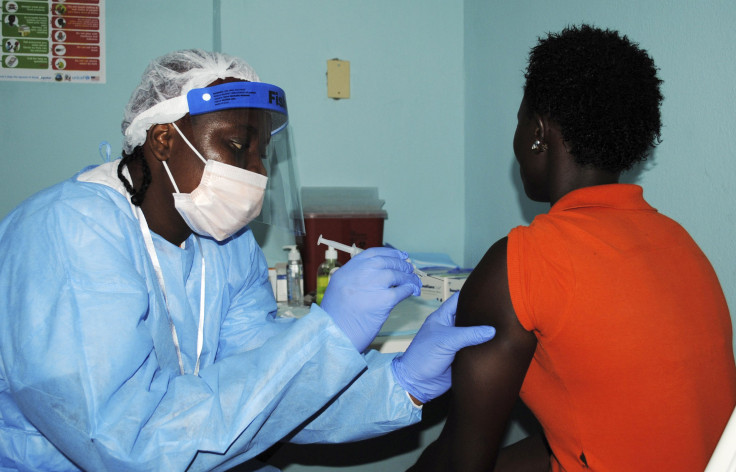
Two experimental Ebola vaccines have passed initial safety tests and are partially through a three-phase clinical trial being conducted in Liberia, the U.S. National Institutes of Health (NIH) said on Thursday. One of the vaccines is from GlaxoSmithKline PLC, and the other from NewLink Genetics Corp.
The two vaccines, administered in a single injection each, are undergoing tests for safety and effectiveness on over 600 people in Liberia, in a mid-stage clinical trial being sponsored by an NIH branch responsible for infectious diseases. Based on the results, the study may advance further, moving on to the next phase of efficacy testing, which would see additional volunteers injected with either the GSK vaccine, the NewLink vaccine, or a control placebo, and then later get assessed to determine whether their body produces antibodies. None of the volunteers are being exposed to the virus, but the immune system’s response is being used as a measure of how effective the vaccine would be in a real-world scenario.
“We are grateful to the Liberian people who volunteered for this important clinical trial and encouraged by the study results seen with the two investigational Ebola vaccine candidates,” National Institute of Allergy and Infectious Diseases Director Anthony S. Fauci said in a statement. “Now we must move forward to adapt and expand the study so that ultimately we can determine whether these experimental vaccines can protect against Ebola virus disease and therefore be used in future Ebola outbreaks.”
Researchers had originally planned to enroll another 27,000 people in Liberia who are considered at risk of Ebola for the trial’s third phase, but there has been only one new confirmed case in the country since mid-February, and cases are declining in Guinea as well, leading to hopes that the epidemic may be winding down, Reuters reported. However, authorities in Sierra Leone have enforced a three-day lockdown in the country, aiming to curb its spread in the country, where dozens of new cases are still being reported every week, BBC reported.
Researchers also say that the virus appears to be mutating less rapidly than once feared. An August study found that Ebola was altering its genetic code about twice as fast as it had in previous outbreaks, leading to worries that it may become airborne, rendering it significantly more deadly. However, a report published Thursday in the journal Science found that the virus’ rate of mutation was within the expected range, and had remained remarkably stable given the widespread human transmissions that have occurred in the most recent outbreak.
The Ebola epidemic, which had spread rapidly since it began in West Africa last year, has killed over 10,200 people, according to the U.S. Centers for Disease Control and Prevention.
© Copyright IBTimes 2024. All rights reserved.





















