US Doctor Groups Debate Best Use Of New Weight-loss Drugs

As powerful new obesity drugs enter the U.S. market, medical associations are keen to advise their members on how to best use them for patients. That is where the debate begins.
Some specialists advocate for broad use of drugs like Novo Nordisk's Wegovy, alongside a healthy diet and exercise. Others recommend prioritizing them for high-risk patients, who have other conditions that are exacerbated by excess weight.
And some are considering more complex, patient-specific assessments to determine the best course of action, according to a Reuters review of existing guidelines and interviews with doctors and executives from six medical associations as they contemplate updating their own guidance.
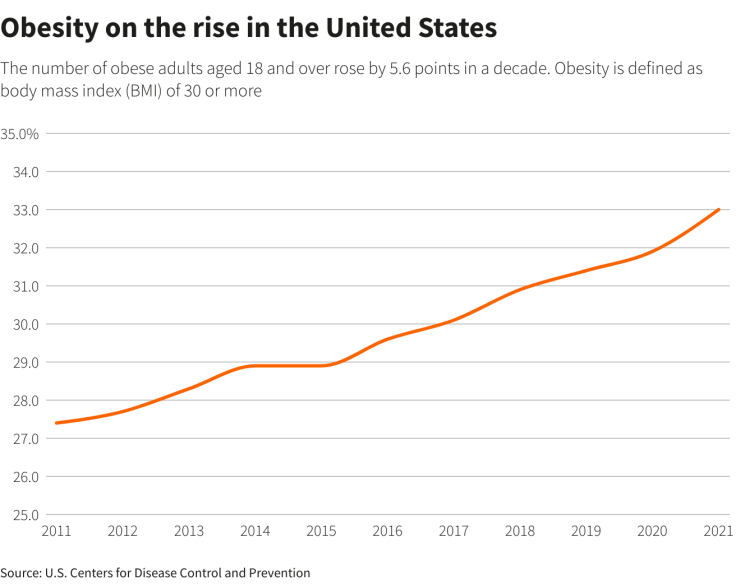
Their recommendations will influence treatment and coverage for nearly 115 million U.S. adults and children who are obese, as well as a significant percentage of overweight Americans who have other health risks such as heart disease or diabetes.
U.S. health insurance companies take into account specialist medical guidelines, among other data, in deciding whether to pay for a treatment. Wegovy, which costs nearly $1,350 a month, has yet to receive wide coverage from private health plans.
Wegovy is approved for use in the United States and Europe, while a similar drug from Eli Lilly and Co known as Mounjaro is expected to receive U.S. approval later this year.
The medications helped patients shed up to 15% and 20% of their weight, respectively, in large clinical trials, more than previously-approved weight-loss treatments.
The clinical trials spanned two years, meaning any longer-term consequences are not yet known, and many patients may need to stay on the drug to keep off the weight.
"These new compounds are game changers, there's no doubt about it," said Anthony Comuzzie, chief executive of The Obesity Society. "But obesity is a complex condition."
The society, whose members comprise many leading U.S. obesity researchers and clinicians, is assembling an expert committee to recommend when to start patients on these drugs and how to integrate that into other types of treatment, Comuzzie told Reuters.
The group last provided obesity treatment guidelines in 2013 alongside the American Heart Association and American College of Cardiology. It aims to issue new recommendations early next year, and plans more frequent updates as data becomes available.
"You may want to use one of those new drugs to achieve the initial treatment, and then move people on to other interventions to maintain what's been achieved," Comuzzie said.
The American Association of Clinical Endocrinology (AACE) said it plans to put out new guidelines on diagnosis, staging, and medical management of obesity in early 2024, having last issued guidance in 2016.
The Endocrine Society plans an update of its weight-loss drug guidelines for summer 2025, 10 years after it last published guidance.
Novo Nordisk and Eli Lilly, which provide some funding to The Obesity Society and the Endocrine Society and partner with AACE, would not say whether they were engaging with medical associations on the creation of new guidelines.
OBESITY AS A DISEASE
The American Medical Association, the nation's largest medical group, recognized obesity as a disease in 2013. Treatment falls under numerous medical specialties, including obesity experts, endocrinologists, gastroenterologists and pediatricians, as well as primary care doctors.
GRAOHIC: Obesity on the rise in the United States -
In interviews, nearly half a dozen obesity specialists shared varying views on how to use Wegovy and similar treatments.
One said she might prescribe the drugs on an adult patient's first visit, if they had tried at least three months of diet and exercise in the past, while another wanted lifestyle interventions for at least six months before turning to drugs.
But some view the new medicines as a genuine breakthrough similar to the introduction of statins, now widely used to lower cholesterol and prevent heart attacks.
"No one at all is worried about the overuse of those," said Dr. Caroline Apovian, co-director of Harvard Medical School's Center for Weight Management who co-authored the previous guidance from The Obesity Society and Endocrine Society.
"In fact, people are claiming that statins should be used by everybody because you want to get the (bad cholesterol) down as low as possible."
There was some concern patients might see the medicines as a quick fix and forego behavioral changes required for the long haul.
"The first and foremost intervention for weight reduction is diet and lifestyle modification," said Dr. Prakash Deedwania, a cardiologist and professor at University of California, San Francisco.
The Obesity Society wants to establish consensus among U.S. experts with its new guidelines and aims to partner with multiple medical groups, including the U.S.-based Endocrine Society and Obesity Canada.
Wegovy is currently ineligible for coverage under the federal Medicare health plan for older Americans. Drugmakers are lobbying Congress to change a law that deems weight-loss drugs as lifestyle therapies that do not require government reimbursement.
Common Wegovy side effects include abdominal pain, nausea and vomiting.
"One of the biggest challenges in obesity medicine is you've got lots of different options that people can potentially pursue," said Dr. Jamy Ard, an obesity researcher at Wake Forest University heading the Obesity Society's guidance project, "and the evidence base for a lot of them is shaky, at best."

© Copyright Thomson Reuters 2024. All rights reserved.





















