Vanadium Dioxide, A Metal That Conducts Electricity But Not Heat
Scientists have discovered “exotic electrical and thermal properties” in a material known as vanadium dioxide which may be applied to develop more efficient thermoelectric systems, according to the findings of a new study to be published in the Jan. 27 issue of the journal Science.
Vanadium dioxide, a dark blue inorganic solid, is known to exhibit odd properties. For instance, the insulator turns into a electricity-conducting metal when it reaches 67 degrees Celsius (152 degrees Fahrenheit), becomes transparent below about 30 degrees Celsius, and absorbs infrared light above 60 degrees Celsius.
But in the recent study, titled “Anomalously low electronic thermal conductivity in metallic vanadium dioxide,” it was found that the material exhibited properties that are exceptions to the Wiedemann-Franz Law which establishes a general relationship between electrical and thermal conductivity. According to the law, good conductors of electricity are generally also good conductors of heat.
Using results from simulations and X-ray scattering experiments, scientists at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) and at the University of California, Berkeley, found the electrons in vanadium dioxide are able to conduct electricity without conducting heat at a rate of thermal conductivity which was “ten times smaller than what would be expected from the Wiedemann-Franz Law.”
“This was a totally unexpected finding… The electrons were moving in unison with each other, much like a fluid, instead of as individual particles like in normal metals,” explained Junqiao Wu, a physicist at Berkeley Lab’s Materials Sciences Division and a UC Berkeley professor of materials science and engineering, according to Berkeley Lab science center.
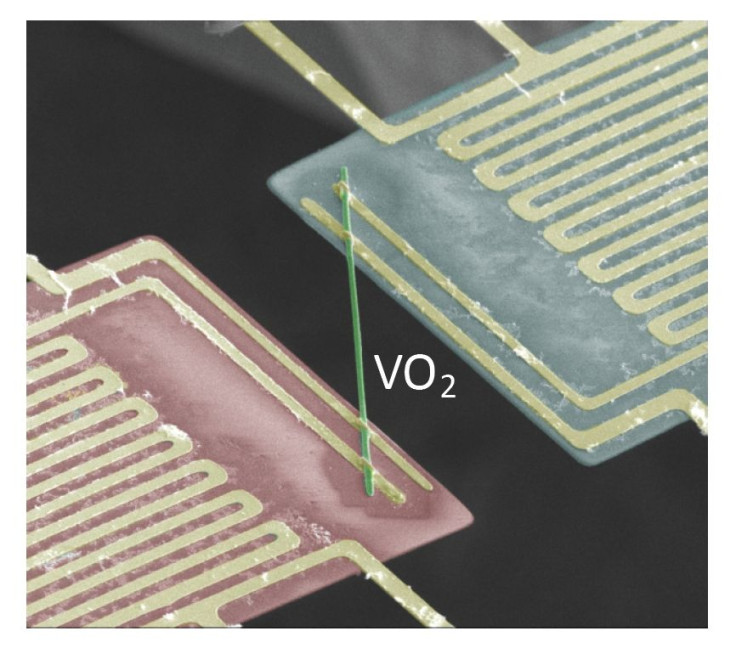
“For electrons, heat is a random motion. Normal metals transport heat efficiently because there are so many different possible microscopic configurations that the individual electrons can jump between. In contrast, the coordinated, marching-band-like motion of electrons in vanadium dioxide is detrimental to heat transfer as there are fewer configurations available for the electrons to hop randomly between,” said Wu.
Most significantly, the study suggests that the amount of heat and electricity the material can conduct can be altered and controlled by mixing it with other materials and the phenomena could result in several applications of thermo-conductivity, such as producing electricity from dissipated heat or developing window coatings with efficient energy transmission.
“This material could be used to help stabilize temperature… By tuning its thermal conductivity, the material can efficiently and automatically dissipate heat in the hot summer because it will have high thermal conductivity, but prevent heat loss in the cold winter because of its low thermal conductivity at lower temperatures,” Fan Yang, the study's co-lead author, said.
© Copyright IBTimes 2025. All rights reserved.




















