A 'Vaccine For The World'? UK Team Seeks Approval For Virus Jab
Scientists behind a coronavirus jab being developed in Britain on Monday hailed it as a potential "vaccine for the world", which could be cheaper to make and easier to store and distribute than its main rivals.
UK pharmaceutical giant AstraZeneca and its partner the University of Oxford said they were seeking regulatory approval for the vaccine after it showed an average 70-percent effectiveness.
That rate jumped to 90 percent depending on the dosage, similar to that in rival vaccines in by Pfizer/BioNTech and Moderna announced last week.
Despite the varying outcomes, AstraZeneca chief executive Pascal Soriot insisted his firm's vaccine would have an "immediate impact" in the fight against the global pandemic.
Nearly 200 million doses will be made before the end of the year and more than 700 million globally by the end of March next year, the company said.

The AstraZeneca/Oxford vaccine could also be stored, transported and handled "at normal refrigerated conditions" of between two and eight degrees Celsius (36-46 Fahrenheit) for at least six months.
That is a far cry from the -70C needed for Pfizer/BioNTech's offering and could allow use of the existing refrigerated supply chain to cut costs.
UK scientific charity the Wellcome Trust called the results "hugely encouraging" and the announcement of data from three separate trials within a year "incredible".
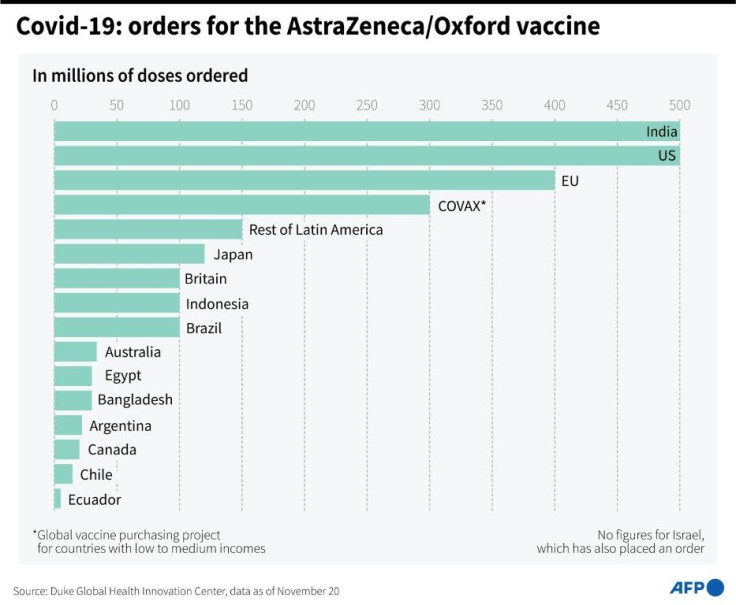
But it said a range of vaccines was needed to protect people around the world, estimating two billion doses were needed to treat high-risk populations in the next 12 months alone.
"Beating Covid-19 will depend on global collaboration," said the trust's head of vaccines, Charlie Weller.
"Promises must be followed by action to ensure equitable distribution and with a significant step-up of investment."
G20 leaders on Sunday said they would "spare no effort" to ensure the fair global distribution of vaccines and support poor countries, whose economies have been hit by the crisis.
But German Chancellor Angela Merkel said she was concerned that no major agreements had yet been struck for poorer nations.
The director of the Oxford Vaccine Group, Andrew Pollard, indicated that its affordable, scalable vaccine could be a game-changer globally.
"We have a vaccine for the world because we've got a vaccine which is highly effective -- it prevents disease and hospitalisation," he told a media briefing.
Questions have been raised about the cost, manufacturing and storage of Pfizer/BioNTech's vaccine.
Requiring temperatures much colder than a standard freezer, there have been concerns about whether lower- and middle-income countries will be able to access it.
By contrast, the AstraZeneca/Oxford jab "could... have a truly significant impact across the globe and enable an end to the Covid-19 pandemic," said infectious diseases specialist Azra Ghani, of Imperial College London.
The partners have also made a "no-profit pledge" that if approved, the vaccine will be affordable and available across the world.
The vaccine showed 90 percent efficacy when given as a half-dose followed by a full-dose at least one month apart, compared with 62 percent when administered as two full doses in the same period.
"We think that by giving a smaller first dose that we're priming the immune system differently, we're setting it up better to respond," said Pollard.
Peter Openshaw, professor of experimental medicine at Imperial College London, said a combination of half and full doses "is great news, potentially increasing the number of people that can be vaccinated and reducing costs".
More than 23,000 adults in the UK and Brazil have taken part in the trials, with the number expected to rise to up to 60,000 thanks to testing also in other countries.
Early results suggested there were 131 cases of Covid-19 among the participants but none was serious.
Britain has been one of the worst-affected countries in the world in the outbreak, registering more than 54,000 deaths from 1.4 million cases.
The government has ordered 100 million doses and hopes to roll out its vaccination programme in the first three months of the year.
© Copyright AFP 2024. All rights reserved.





















