EU Looks At Ramping Up COVID Vaccinations Of Kids, Developing Antivirals
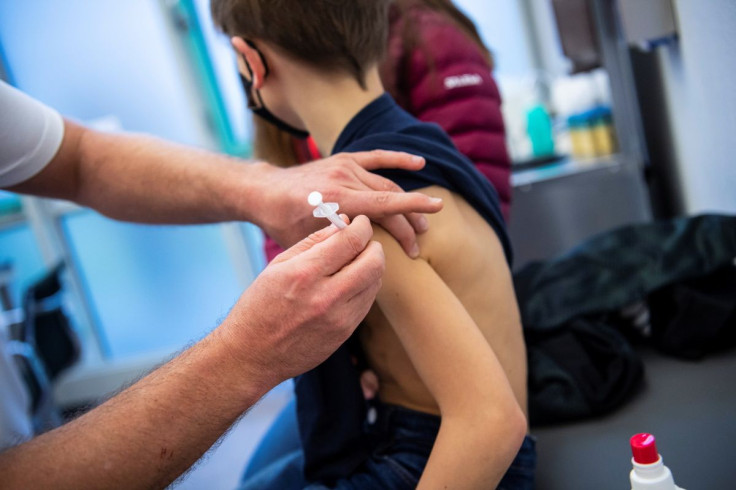
European Union governments should ramp up COVID-19 immunisations of children, the European Commission said on Wednesday in presenting its strategy to move away from the emergency phase of the pandemic, which includes plans to develop antivirals.
With a drop of cases and deaths linked to COVID-19, the EU has entered a new stage of the pandemic in which mass testing and mass reporting of cases are no longer required, EU health commissioner Stella Kyriakides said, confirming what Reuters reported on Tuesday.
But new COVID-19 surges are likely as the virus is expected to continue mutating, and therefore countries should have in place plans to shift back to emergency mode, and should ramp up vaccinations, the commission said.
In a document outlining the strategy for the post-emergency phase of the pandemic, Brussels urged governments to continue pushing for the immunisation of the unvaccinated, especially children before the start of next school season in the autumn.
Immunisations are below 15% among children between 5 and 9, the youngest age group for which COVID-19 vaccines have been authorised in Europe. That compares to over 70% of teens aged 15 to 17, the document says.
The Commission also said it could back the development of new drugs against COVID-19, especially antivirals that are easier to store and administer.
The EU "will explore possibilities to support projects targeting the development of antivirals," it said.
Antiviral pills against COVID-19 developed by Pfizer and Merck & Co have been approved for use in the EU. But their uptake has so far been limited, due to a range of reasons including the slowing of the pandemic, high prices and complicated national procedures to prescribe them.
The EU executive also said it would work to support the development of the next generation of COVID-19 vaccines which it expects will offer more robust and longer-lasting protection against infection or transmission.
© Copyright Thomson Reuters 2024. All rights reserved.





















