Experimental Ebola Drug-Maker Chimerix Stock Falls After Dallas Ebola Patient Dies
Stock in the little-known American biopharmaceutical company Chimerix took an immediate hit Wednesday morning after the death of Thomas Eric Duncan, the first person diagnosed with Ebola in the United States. He was being treated at a Dallas hospital with an experimental drug from Chimerix.
Since the Ebola outbreak began in West Africa, a handful of small U.S. biotech firms have seen their shares steadily rise as investors seize on research into the deadly disease. The stock runup intensified when officials announced that Duncan had been admitted to a Dallas hospital on Sept. 30.
Chimerix’s stock fell nearly 12 percent to $29.21 in Wednesday morning’s Nasdaq trading after the announcement of Duncan's death. Shortly after a Dallas hospital said Monday that it would treat him with Chimerix's drug brincidofovir, its stock jumped.
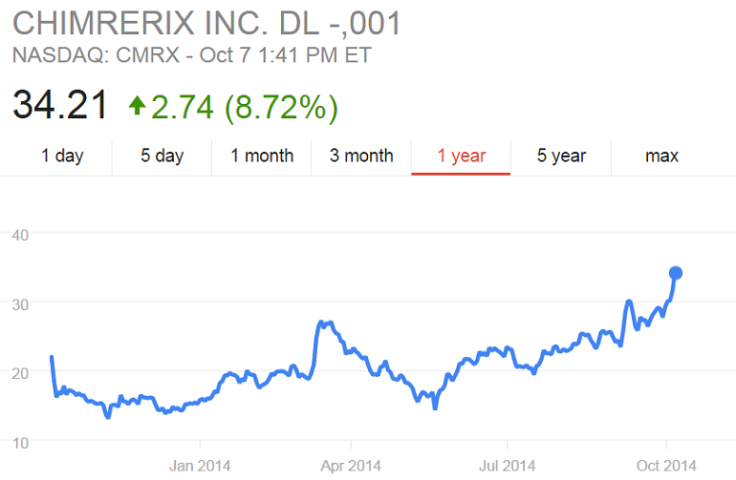
The Durham, North Carolina-based biopharmaceutical company hasn’t been one of the major players on the Ebola landscape, eclipsed by others such as Tekmira, which produced the TKM-Ebola treatment that’s also been used on patients.
Since 2011, Chimerix has received more than $22 million from the Biomedical Advanced Research and Development Authority to develop the drug in question, also known as CMX001. At the time the government was investigating whether it could be used as a “countermeasure in the event of a smallpox release.” The money added to another $37 million Chimerix had received from the National Institute of Allergy and Infectious Diseases and from private investors.
On Sept. 2, the company announced another $17.0 million award for trials. On Monday, Chimerix announced that the U.S. Food and Drug Administration granted “Emergency Investigational New Drug Applications,” to use brincidofovir for Ebola, in answer to the requests of treating physicians. And the Texas Health Presbyterian Hospital Dallas announced that it would use the drug to treat Thomas Eric Duncan, the Ebola patient.
“Data collected over years of clinical development of brincidofovir have allowed us to progress this compound into Phase 3 programs,” the company said in a press release, adding that the research “provided information on the safety and dosing of brincidofovir to allow it to be explored as a potential therapy for Ebola virus disease.”
While there are a handful of drugmakers that have taken the lead on the Ebola fight, Chimerix isn’t one of them. And experts say there are more promising treatment options available.
“It’s a bit surprising,” said Dirk Haussecker, an investor and consultant in the RNAi therapeutics industry, noting that another drug, made by Canadian firm Tekmira, has already been successfully tested on animals and is currently in human trials, while the Chimerix drug has only been tested on cell cultures.
“That is great, but we have many drugs that inhibit the replication of Ebola in cell culture,” said Tom Geisbert, a virologist at the University of Texas Medical Branch who has worked on Ebola vaccine and treatment research. “What we don’t have is many drugs that can completely protect monkeys against Ebola,” he said, noting that only Tekmira's drug and ZMapp have proven effective.
Tekmira Pharmaceuticals has become one of the most important drug developers in the Ebola fight. Doctors used the company’s drug, TKM-Ebola, to treat at least three patients, including American aid worker Rick Sacra, who was treated at a Nebraska hospital and later recovered.
Like most other companies in the Ebola treatment business, the Vancouver, Canada-based firm has been working with the U.S. government, which has spent millions in grants and contracts to fund research for diseases like Ebola, which it feared could be used as a biological weapon. In 2010, it signed a $140 million contract with the Department of Defense for the research. In January, researchers dosed their first human subjects in a clinical trial.
This year, Tekmira stock has surged more than 200 percent, with a 18.2 percent increase the day news broke about the American patient.
Another experimental drug called ZMapp, made by private firm Mapp Biopharmaceutical in San Diego, successfully treated earlier Ebola patients, including two U.S. aid workers. But ZMapp supplies have since run out.
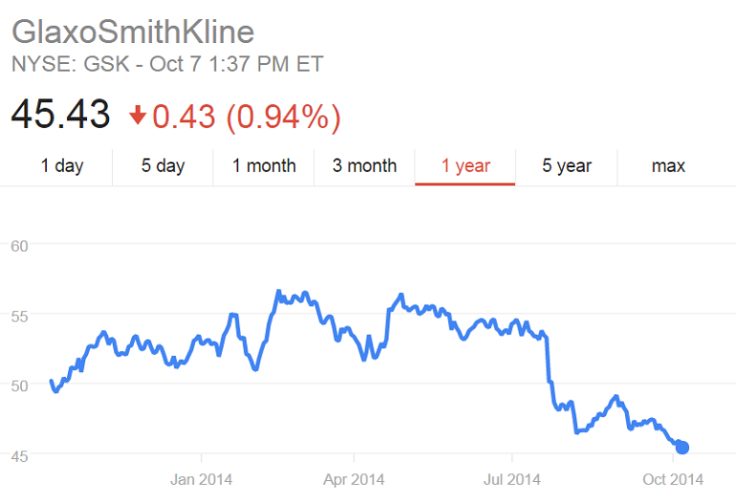
In late August, GlaxoSmithKline saw a slight jump in its stock after it began testing a vaccine candidate, known as NIAID/GSK Ebola Vaccine, on human subjects. The preventive vaccine was one of many GSK took on when it acquired Okairos, a small Italian biotech firm, in 2013.
While GSK shares were strengthened during the first part of the Ebola outbreak, its stock has fallen in recent weeks.
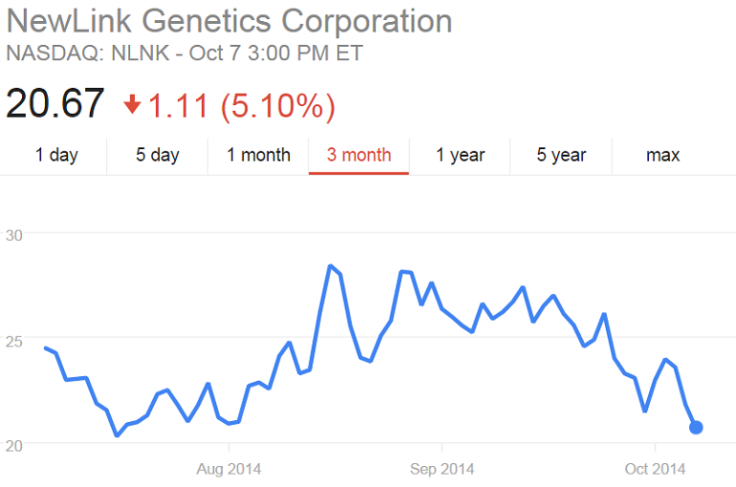
Shares of NewLink Genetics Corp. experienced a boost in March when the outbreak started, but have since fallen back to previous levels. The American biotech company licensed an Ebola vaccine developed at the Canada National Microbiology Laboratory, and has received funding from the U.S. government’s Biomedical Advanced Research and Development Authority. In August, its stock jumped 36 percent after it announced plans for human trials but has since fallen again.
© Copyright IBTimes 2024. All rights reserved.






















