FDA Warns Of Missed Alerts On Phone-Connected Diabetes Devices, Leading To 'Serious Harm'
It blames software updates, 'Do Not Disturb' mode, or connected devices that alter notification settings
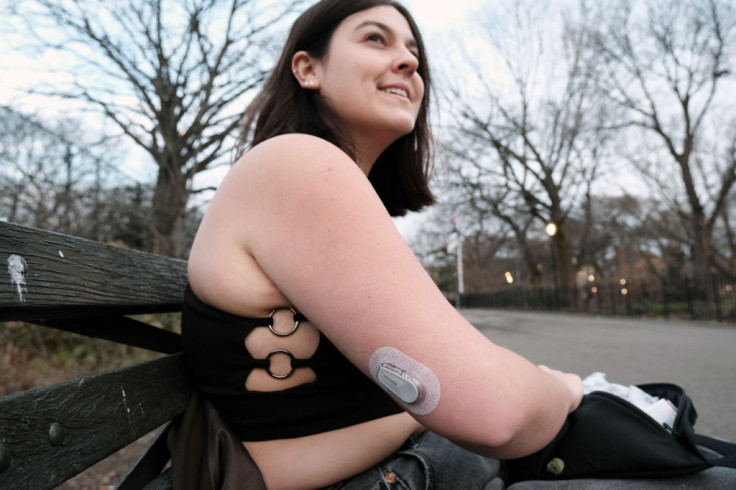
The Food and Drug Administration has issued an alert warning diabetes patients and caregivers about potential issues with smartphone alerts for continuous glucose monitors (CGMs).
The warning applies to insulin pumps and other diabetes-related devices, according to the FDA.
Courtney Lias, a director at the Office of In Vitro Diagnostic Products, warned consumers to review their smartphone settings.
"Users should stay aware of alert settings and monitor these devices to ensure they continue to receive critical alerts as expected," said Lias.
The agency reports that missed alerts due to smartphone settings or updates "may have contributed to serious harm," including severe hypoglycemia, hyperglycemia, diabetic ketoacidosis, or even death.
Common causes of missed alerts include software updates, "Do Not Disturb" mode, or connected devices like Bluetooth headphones that alter notification settings.
The FDA urged users to regularly check their smartphone settings, especially after updates or hardware changes, to ensure alerts function properly.
Patients should verify compatibility before updating their operating system.
Patients can contact device manufacturers if notification alerts are not working as expected for more help.
The FDA is collaborating with medical device manufacturers to improve smartphone compatibility.
The agency urges users to report issues through the MedWatch Voluntary Reporting Form.
© Copyright IBTimes 2024. All rights reserved.





















