Improving Lithium-Ion Batteries: Tweaking Solid-State Electrolytes Could Make Them Safer, Stronger

A team of researchers from Virginia Commonwealth University are making inroads into lithium-ion battery technology by focusing research on improving the conductivity and safety in these devices, according to a paper in the journal Proceedings of the National Academy of Sciences.
The lithium-ion invasion is finally complete. They are seemingly everywhere. They power all our devices from cameras, smartphones, tablets to pacemaker systems and satellites. But, in the recent past, they have gained a notorious reputation for being unstable and volatile. Reports of smartphone batteries exploding have led to them being a restricted item in air spaces. According to the United Parcel Service website, customers are required to sign a UPS Dangerous Goods service agreement before shipping lithium-ion or lithium metal batteries. Equipment without batteries can be transported by air.
Most lithium-ion batteries use liquid-state electrolytes that help carry charges from one battery electrode to another. They are extremely unstable compared to solid-state electrolytes but are preferred for their superior conductivity. The team is looking to establish a finer balance between safety and conductivity to improve the function of lithium-ion batteries.
The study by the team has shown it is possible to design solid-state electrolytes not only to be as conductive as their liquid counterparts but also very stable, said a news release on the Virginia Commonwealth University website.
"Theoretically, you can have your cake and eat it too, when it comes to the stability and conductivity," Puru Jena, a distinguished professor in the Department of Physics in the College of Humanities and Sciences, was quoted as saying in the release.
Electrolytes, which are central to a battery, are salts composed of positive and negative ions. Electrolytes contain both positive and negative ions. A lithium-ion battery works by creating a flow of positive lithium ions between two electrodes via an electrolyte. "Lithium ions can flow freely through liquid-state electrolytes but are less mobile in a solid-state electrolyte, which adversely affects conductivity," said the release.
The researchers produced a model in which a single negative ion from the solid-state electrolyte is replaced with a negative cluster ion. A negative cluster ion is made of a collection of atoms with more electrons than protons. They replace the absent ion in the electrolyte.
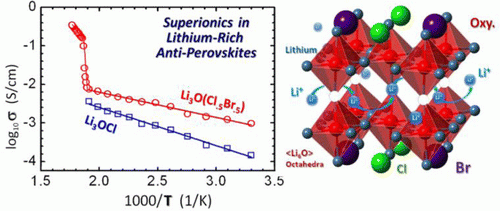
According to the release, "the electrolyte, which belongs to a family of crystals called antiperovskites, contained positive ions made of three lithium atoms and one oxygen atom. The positive ions were joined with a single chlorine atom that was a negative ion." This was the composition of the solid-state electrolyte used in the model.
When the chlorine atom in the electrolyte was replaced by a negative cluster ion created by one boron atom and four fluorine atoms, it helped the lithium ions to flow easily.
"Replacing the chlorine ion with cluster ions improves conductivity because these ions are larger and allow the lithium ions to move quickly, as if they were in a liquid," said Hong Fang, a postdoctoral fellow in the Department of Physics and part of the team.
Other combinations of negative cluster ions were identified to potentially enhance conductivity.
The team hopes that their computational model can be tested by companies to provide a definitive way this technology can be imbibed into lithium-ion battery manufacturing.
© Copyright IBTimes 2024. All rights reserved.





















