New Russian Virus Vaccine One Among Many
With Russia's announcement Tuesday that it had become the first country to approve a vaccine against COVID-19, here is a look at the more than two dozen other candidates currently in development.
Russian President Vladimir Putin shocked the international community by claiming the new vaccine -- dubbed "Sputnik V" after the Soviet satellite -- conferred "sustainable immunity" against the novel coronavirus.
He said that one of his daughters had been inoculated with the vaccine, developed by the Gamaleya research institute and the Russian defence ministry.
A vector vaccine -- meaning it employes another virus to carry the immune response into human cells -- Sputnik is based on similar technology to a Chinese prototype.
Deputy Prime Minister Tatyana Golikova said she hoped the vaccinations could begin within weeks, but details about the process were scant.
Last week the World Health Organization urged Moscow to follow established guidelines and "go through all the stages" needed to develop a safe vaccine.
Eleanor Riley, professor of Immunology and Infectious Disease at the University of Edinburgh, said larger, phase 3 trials were needed for Sputnik.
Phase 3 trials test the safety and efficacy of new medicines and can last several years.
"But there is a big difference between a large vaccine trial (with careful and frequent follow up of all vaccinated individuals) and deployment of a vaccine to the general public," said Riley.
Francois Balloux, professor of Computational Systems Biology at University College London went as far as to dub Russia's announcement "reckless and foolish".
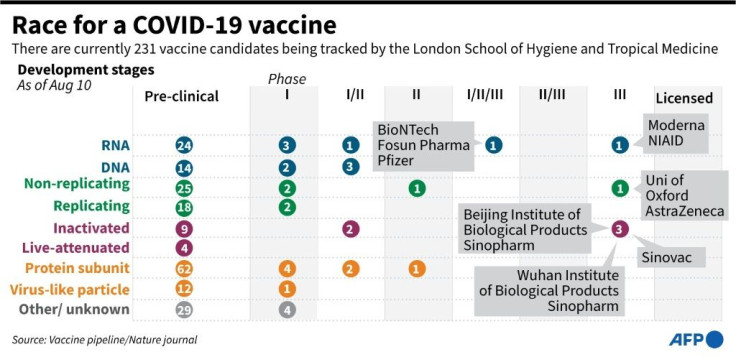
The WHO says there are 26 "vaccine candidates" currently undergoing clinical trials worldwide.
Several are in the final phase 3 stage, whereby developers -- testing on large cohorts, up to tens of thousands of people -- monitor for efficacy and potential toxicity prior to eventual submission for approval.
These include a European project being developed at Oxford University in tandem with AstraZeneca and a Chinese variant from biopharmaceutical company Sinovac in collaboration with Brazilian research institute Butantan.
The latter is being tested on 9,000 health professionals in Brazil.
A vaccine developed by Germany's BioNTech and US pharma giant Pfizer entered phase 3 last month, with the companies planning to test it on 30,000 young volunteers.
The American firm Moderna also says it plans to trial its vaccine among 30,000 people.
Since mid-July China's Sinopharm has begun testing its candidate on 15,000 people in the United Arab Emirates.
Beyond the tests already under way, the WHO is monitoring a further 139 potential vaccines which are still at the pre-clinical evaluation stage, involving testing in the lab or on animals.
US biotech company Novavax said last week its experimental COVID-19 vaccine had elicited a robust immune response, producing more antibodies than are present in recovered patients.
It said the candidate vaccine was generally well tolerated among volunteers.
The final stage Phase 3 trial of the product, called NVX-CoV2373, is set to take place this autumn.
The European Union has ordered 300 million doses of potential vaccine from French drug producer Sanofi, and the US has said it will pay $2.1 billion for its development.
Britain has also ordered 60 million doses of the Sanofi vaccine, developed in conjunction with GSK.
© Copyright AFP 2024. All rights reserved.





















