Plan B: Suit Wants Morning After Pill Available to Girls
NEW YORK (Reuters) - Reproductive rights advocates on Wednesday asked a federal judge in Brooklyn to make the morning-after pill immediately available to girls of all ages without a prescription.
The move by a coalition headed by the Center for Reproductive Rights attempts to override a decision by U.S. Secretary for Health and Human Services Kathleen Sebelius that maintains the requirement of a prescription for girls age 16 and younger.
The group asked to add Sebelius as a defendant to an existing federal lawsuit that has been dormant for six years and was reawakened by Sebelius's unprecedented move two months ago to restrict the availability of Plan B emergency contraceptives for minors.
Sebelius is also at the center of another birth control controversy in which the same advocates applaud her decision to require health insurance plans to offer birth control free of charge, even for Roman Catholic universities and charities.
The coalition filed motions asking U.S. District Judge Edward Korman for permission to formally reopen a lawsuit first brought in 2005 challenging the age restriction. The coalition is also seeking an order immediately lifting both age and point-of-sale restrictions on Plan B and equivalent emergency contraceptives.
After 10 years of stalling and putting politics before science, it's time to bring emergency contraceptives out from behind the pharmacy counter, Center for Reproductive Rights President Nancy Northup told reporters in a conference call.
The center's efforts to restart the litigation come less than two months after Sebelius instructed the U.S. Food and Drug Administration to deny a petition by drug-maker Teva Pharmaceutical Industries Ltd to make its one-pill version of Plan B available over-the-counter to women and girls of all ages.
In doing so, Sebelius overruled the FDA's recommendation that the petition be granted, saying she did not believe there was sufficient evidence that girls younger than 17 could properly use the drug.
It was the first time in agency history the HHS overruled a scientific recommendation from the FDA, prompting critics to question whether the decision was based on inadequate scientific evidence or politics.
The path towards OTC status for post-coital contraception has been unnecessarily complicated by political interference, the plaintiffs wrote in court papers.
An article that ran in the January 12 issue of the New England Journal of Medicine appeared to provide support for that contention, saying scientific data showed girls as young as 15 could safely use emergency contraceptives without a doctor's supervision.
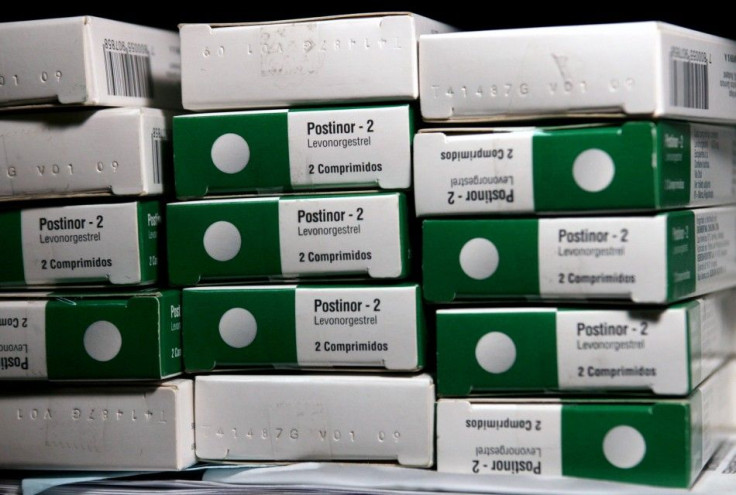
Both the one- and two-pill versions of Plan B and the generic equivalents contain the drug levonorgestrel. If taken up to 72 hours after unprotected sex, Plan B has proven up to 89 percent effective at preventing pregnancy.
Currently, only women age 17 or older can obtain the drug without a prescription. However, point-of-sale restrictions require that all women present identification to a pharmacist before obtaining the drug.
Representatives for HHS and for the U.S. Attorney's Office in the Eastern District of New York, which represents the FDA, declined to comment.
An FDA spokesperson did not immediately return requests for comment on Wednesday.
(Editing by Barbara Goldberg and Daniel Trotta)
© Copyright Thomson Reuters 2024. All rights reserved.






















