Covid-19 Vaccine Trial Paused After Unexplained Illness
Clinical trials on one of the most advanced experimental Covid-19 vaccines, which is being developed by pharmaceutical company AstraZeneca and Oxford University, were "paused" Tuesday after a volunteer developed an unexplained illness.
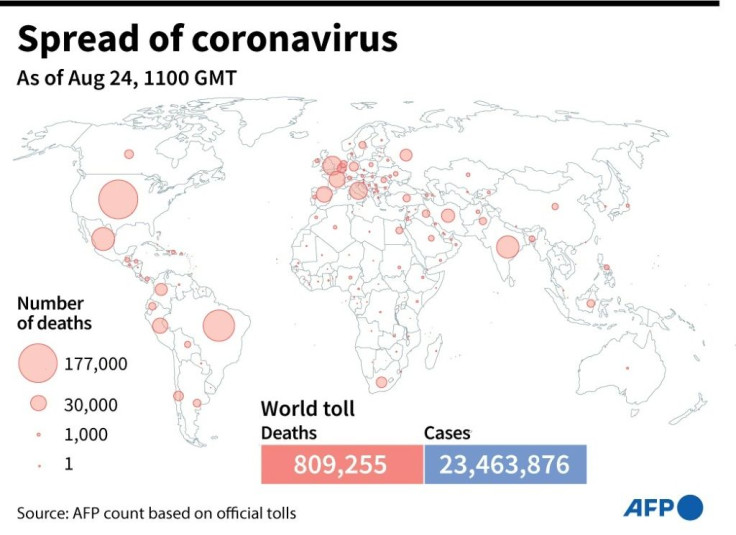
With billions of people around the world still suffering with the fallout of the pandemic and the global death toll nearing 900,000, a worldwide race for a vaccine is underway, with nine companies already in late-stage Phase 3 trials.

Worldwide infections to date now stand at more than 27 million, and more than 890,000 people have died from the disease.
Russia has already approved a vaccine, and research published in The Lancet medical journal last week said patients involved in early tests developed antibodies with "no serious adverse events", although scientists cautioned the trials were too small.
A spokesperson for the AstraZeneca vaccine said in a statement "we voluntarily paused vaccination to allow review of safety data by an independent committee".
"This is a routine action which has to happen whenever there is a potentially unexplained illness in one of the trials, while it is investigated, ensuring we maintain the integrity of the trials."

The company said that in large-scale trials, illnesses will sometimes happen by chance, but must be reviewed independently.
AstraZeneca did not offer further details, but David Lo, a professor of biomedical sciences at the University of California, Riverside, told AFP the pause may not necessarily be a setback.

"Probably right now it's just being cautious -- it's a pause, it's not the same thing as saying, 'We can't move forward'," said Lo.
"In some ways I'm quite relieved, it means they're really paying attention."

The volunteer may have experienced an adverse reaction already seen in earlier patients such as fever and soreness, but in a more severe form, Lo added.

Britain's health minister Matt Hancock said it was not the first pause in the trials of the AstraZeneca vaccine.

"It's a standard process in clinical trials. There was a pause earlier in the summer and that was resolved without a problem," he told Sky News.
China, meanwhile, put its homegrown vaccines on display for the first time at a Beijing trade fair this week, and authorities hope it will be approved for use by the end of the year.
The economic fallout from the virus continues to wreak havoc on economies around the world, with governments desperate to get back to normality.
South Africa announced its economy had shrunk by more than half in the second quarter, as the epidemic took its toll on Africa's most industrialised state.
Latin America and the Caribbean surpassed 300,000 virus deaths. Argentina's caseload surpassed the half-million mark, while in Peru, which has the highest per capita coronavirus death rate in the world, fatalities climbed beyond 30,000.
European countries were battling with high-profile sporting and political fallout from the pandemic on Tuesday.
Tour de France director Christian Prudhomme has tested positive for the virus, prompting French Prime Minister Jean Castex to take a test, officials said, after the pair shared the same car to follow a stage of the cycling event.
France's football team, meanwhile, was forced to take to the field against Croatia on Tuesday without star striker Kylian Mbappe after he tested positive.
The French Open tennis tournament suffered a blow after world number one and defending champion Ashleigh Barty announced she would not take part.
His doctor, however, was optimistic.
"All monitored parameters... are reassuring," said Alberto Zangrillo, adding that Berlusconi's medical condition was in "constant favourable evolution".
© Copyright AFP 2024. All rights reserved.





















