As Vaccines Are Rolled Out, Questions Remain
European countries are following the United States, Britain and a handful of other countries to begin rolling out coronavirus vaccines.
The rapid development and approval of drugs has been hailed the world over, questions remain about the availability, effectiveness and side effects of the jabs.
It usually takes roughly 10 years to develop and market a new vaccine, but the process was vastly accelerated for Covid-19.
A vaccine developed by the American company Pfizer and the German company BioNTech was approved for use in Britain on December 2. Thousands of older people have since received the first dose.
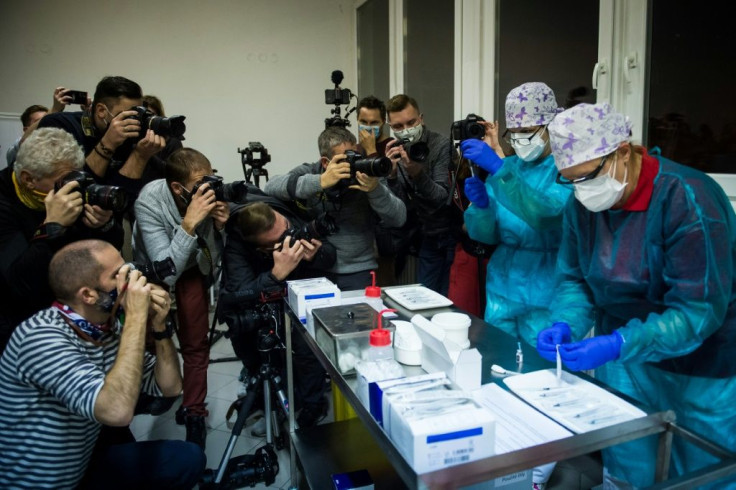
A total of 16 countries and the European Union have given the green light to the Pfizer-BioNTech vaccine.
The US Food and Drug Administration granted emergency authorisations to the Pfizer-BioNTech drug and another jab from the American company Moderna.
Russia began vaccinations on December 5 with its domestic drug Sputnik V, which is still in its third phase of clinical trials. China has already given the go-ahead for emergency use of some of their vaccines, even though none have yet been formally approved.

A total of 16 vaccines are in the final stage of development, including those already on the market, the World Health Organization says.
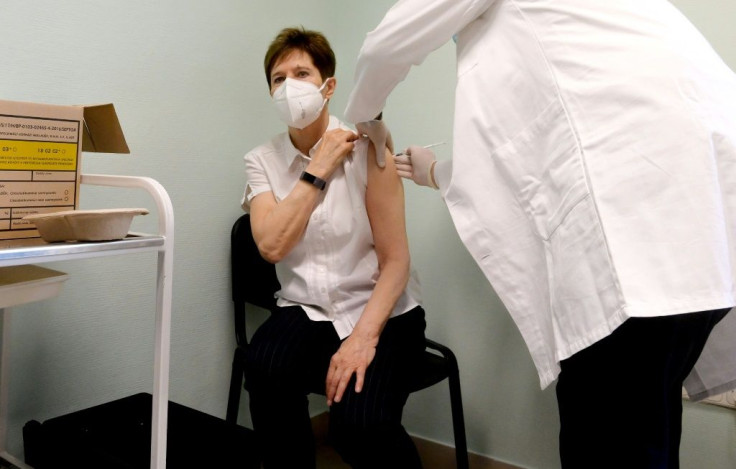
Vaccinations can begin from Sunday following approval of the Pfizer-BioNTech jab by the European Medicines Agency (EMA).
Each member country will take the lead in defining their priorities with the rollout.
But three member states -- Germany, Hungary and Slovakia -- started vaccinations a day early on Saturday.

Since 9 November, four manufacturers have announced that their vaccine is effective: Pfizer-BioNTech, Moderna, the British alliance AstraZeneca-University of Oxford and the Russian state institute Gamaleia.
These announcements are based on phase 3 clinical trials that involve tens of thousands of volunteers.

However, detailed and validated data are available only for the Pfizer-BioNTech and AstraZeneca-Oxford drugs.
The scientific journal The Lancet confirmed on December 8 that AstraZeneca's vaccine was 70 percent effective on average.
The FDA confirmed the Pfizer-BioNTech vaccine at 95 percent efficacy with Moderna claiming 94.1 percent for its drug. Russia claims a 91.4 percent efficacy for its Sputnik V vaccine.
The AstraZeneca-Oxford vaccine is the least expensive at around EUR2.50 per dose). The vaccines from Moderna and Pfizer/BioNTech have a logistical handicap, as they can only be stored over the long term at very low temperatures (-20? Celsius for the former, -70?C for the latter).
Experts insist that with clinical trials carried out on tens of thousands of volunteers, any major risk would have already been detected. But rarer side effects, or those affecting specific patient profiles, cannot be ruled out.
According to the FDA, the Pfizer-BioNTech vaccine can result in painful reactions on the arm where the injection is made. Other undesirable side effects include fatigue, headaches, cramps and, more rarely, fever.
The most important is the long-term efficacy.
Penny Ward of King's College in London said key questions were how long would protection last and could the virus eventually mutate and no longer be covered by the vaccine?
Another crucial question is whether the vaccines act differently in populations most at risk, starting with elderly people who are more likely to develop a serious form of Covid-19.
It also remains to be seen whether these vaccines block the transmission of the virus, and also reduce the severity of the disease in those who have received the jab.
European Union experts believe that the current vaccines against Covid-19 remain effective against the new strain of the virus detected in Britain and elsewhere, which is thought to be more infectious.
"At the moment there is no evidence to suggest" that the Pfizer-BioNTech vaccine "is not effective against the new variant", the European Medicines Agency said.
Co-director of the German laboratory BioNTech, Ugur Sahin, echoed that message, adding that his company would in any case be in a position to provide a vaccine for a new strain of Covid-19 within six weeks.
© Copyright AFP 2024. All rights reserved.




















